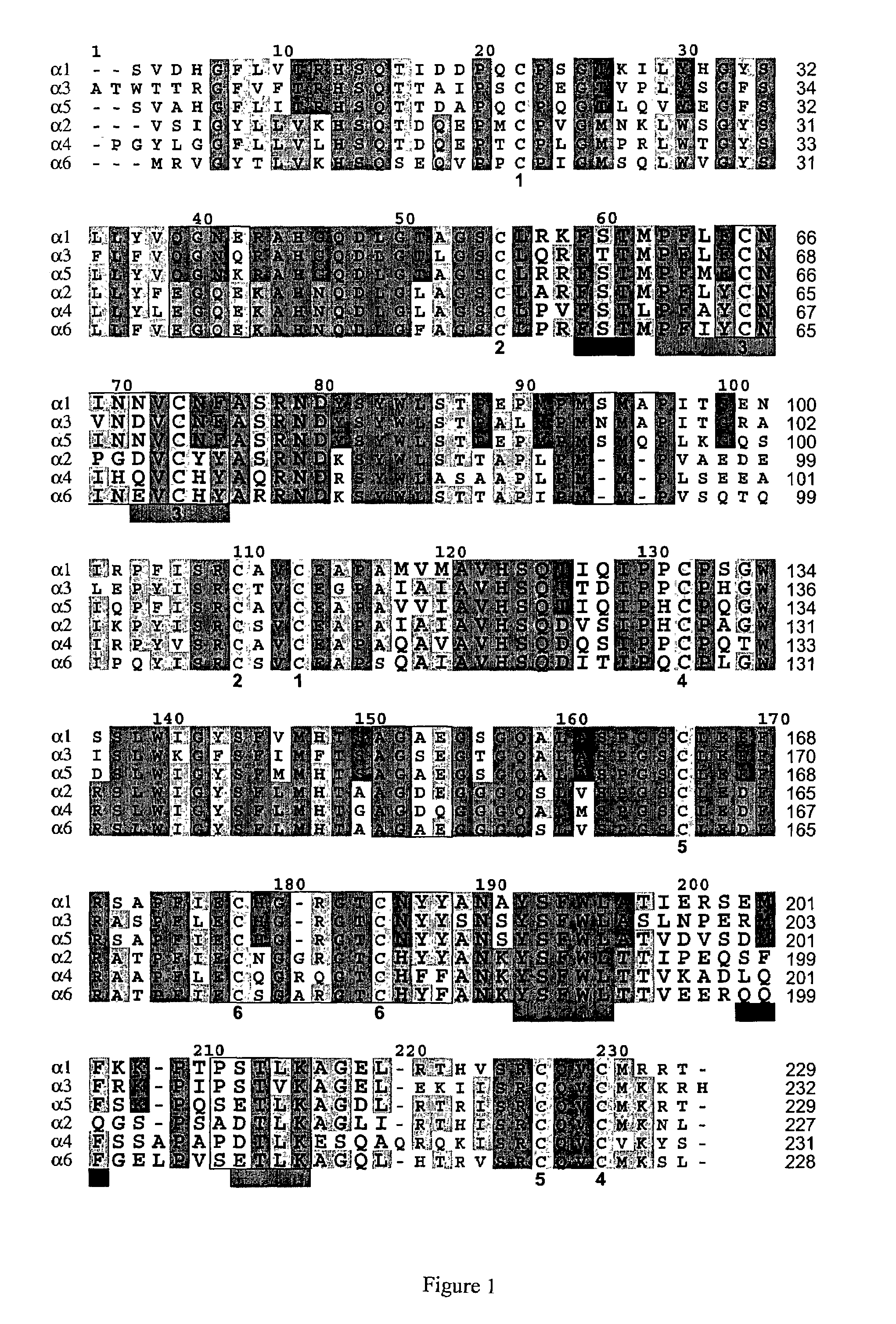
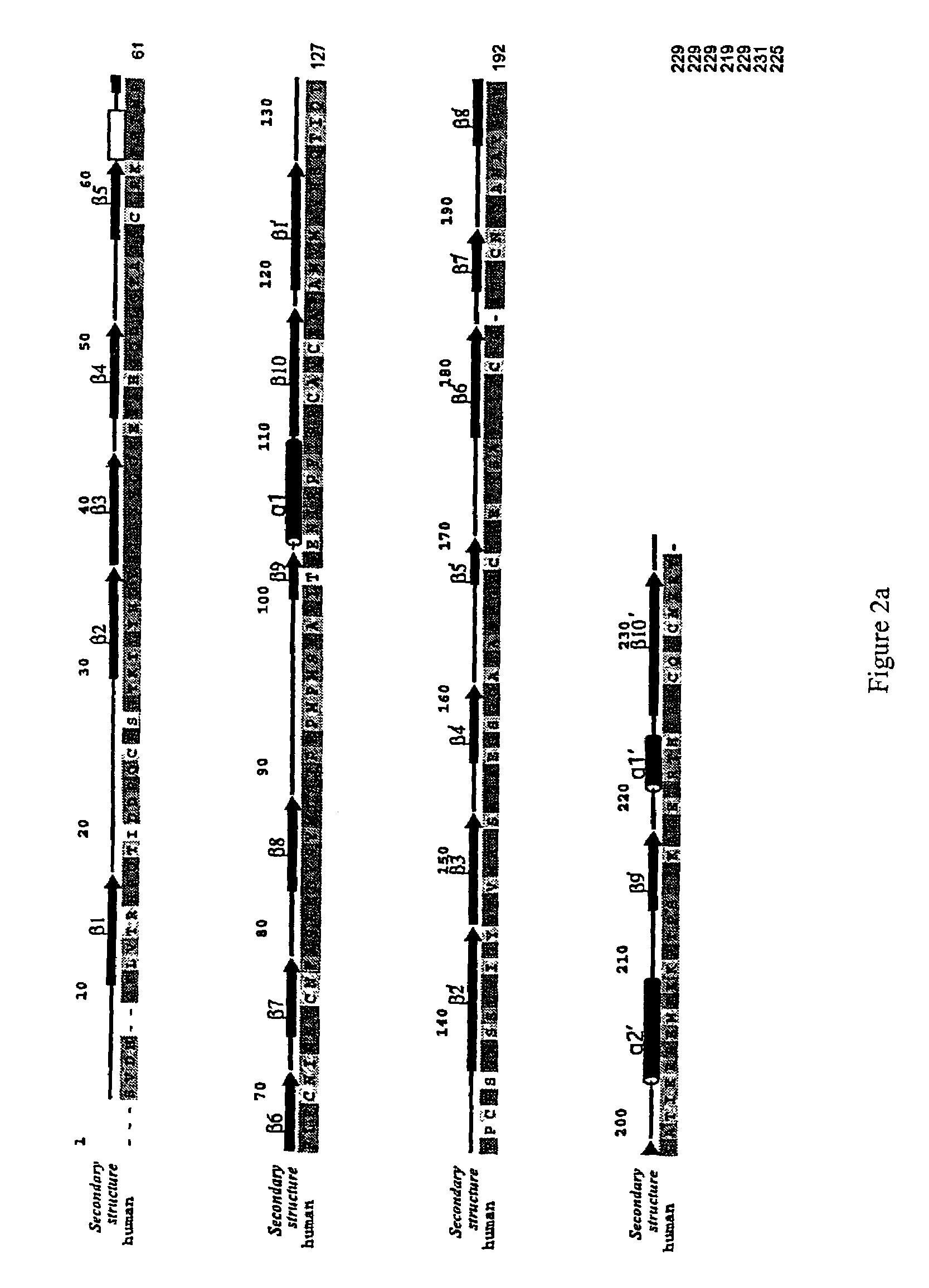
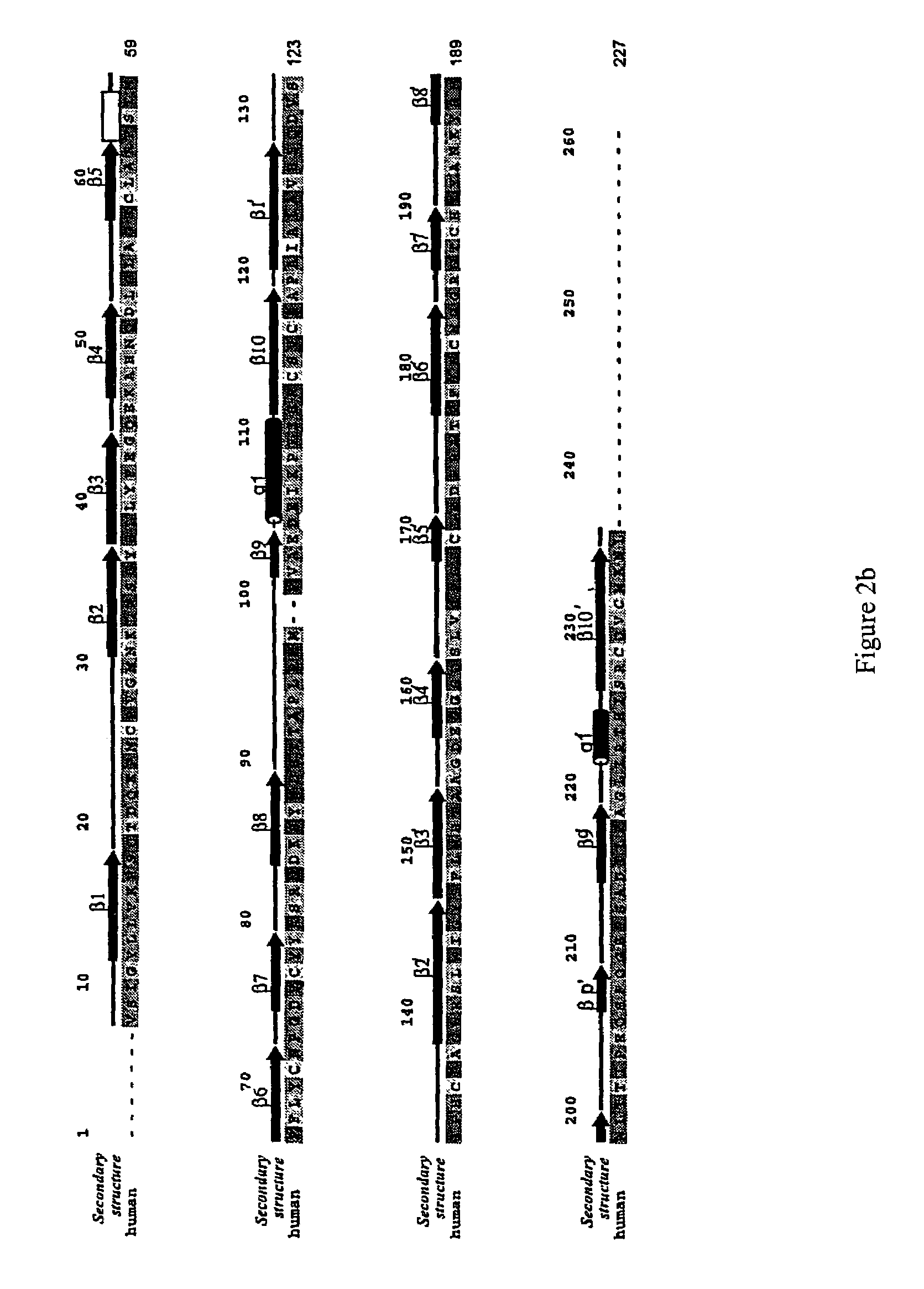
Crystallized structure of type IV collagen NC1 domain hexamer
a collagen nc1 and crystal structure technology, applied in the field of crystal structure, can solve the problems of large number of growth factors known to play a role in angiogenesis and growth factor antagonists may only have limited use in cancer treatment, and achieve the effect of inhibiting angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0337]Protein Purification and Crystallization. The [(α1)2.α2]2 NC1 hexamer was isolated from bovine eye lenses purchased from Pel-Freeze Biologicals (Rogers, Ark.) (37). Briefly, LBM was prepared by sonication of the lenses in the presence of 1 M NaCl and protease inhibitors (38). To cleave the NC1 domain from the full-length type IV collagen, the LBM preparation was digested with bacterial collagenase at 37° C. The NC1 hexamer was purified by using DE-52 and S-300 column chromatography.
[0338]Initial crystallization screening with commercial sparse matrix kits (Hampton Research, Laguna Niguel, Calif.) was carried out using concentrated protein (10 mg / ml) and hanging drop vapor diffusion method. LBM NC1 crystals grow as small clusters overnight in 10% (w / v) PEG 20K, 0.1 M Bicine buffer (pH 9.0) at room temperature. Diffraction quality crystals were grown using microseeding procedures under similar conditions with lower protein concentration. The crystals belong to monoclinic P21 spa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap