Human rotavirus and vaccine composition comprising same
a technology of human rotavirus and vaccine composition, which is applied in the field of human rotavirus, can solve the problems of not easy to develop a new vaccine, and the cancellation of rotashield®
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of Human Rotaviruses
Identification of P Genotype of Rotaviruses Isolated from Stool Specimens
[0046]Stool specimens obtained from 452 children infected by rotavirus and hospitalized in Chung-Ang University Yongsan Hospital in Seoul, South Korea were subjected to seminested multiplex PCR (Gouvea et al., J Clin. Microbiol. 28:276-282, 1990; 32:1338-1340, 1994) to identify P genotype.
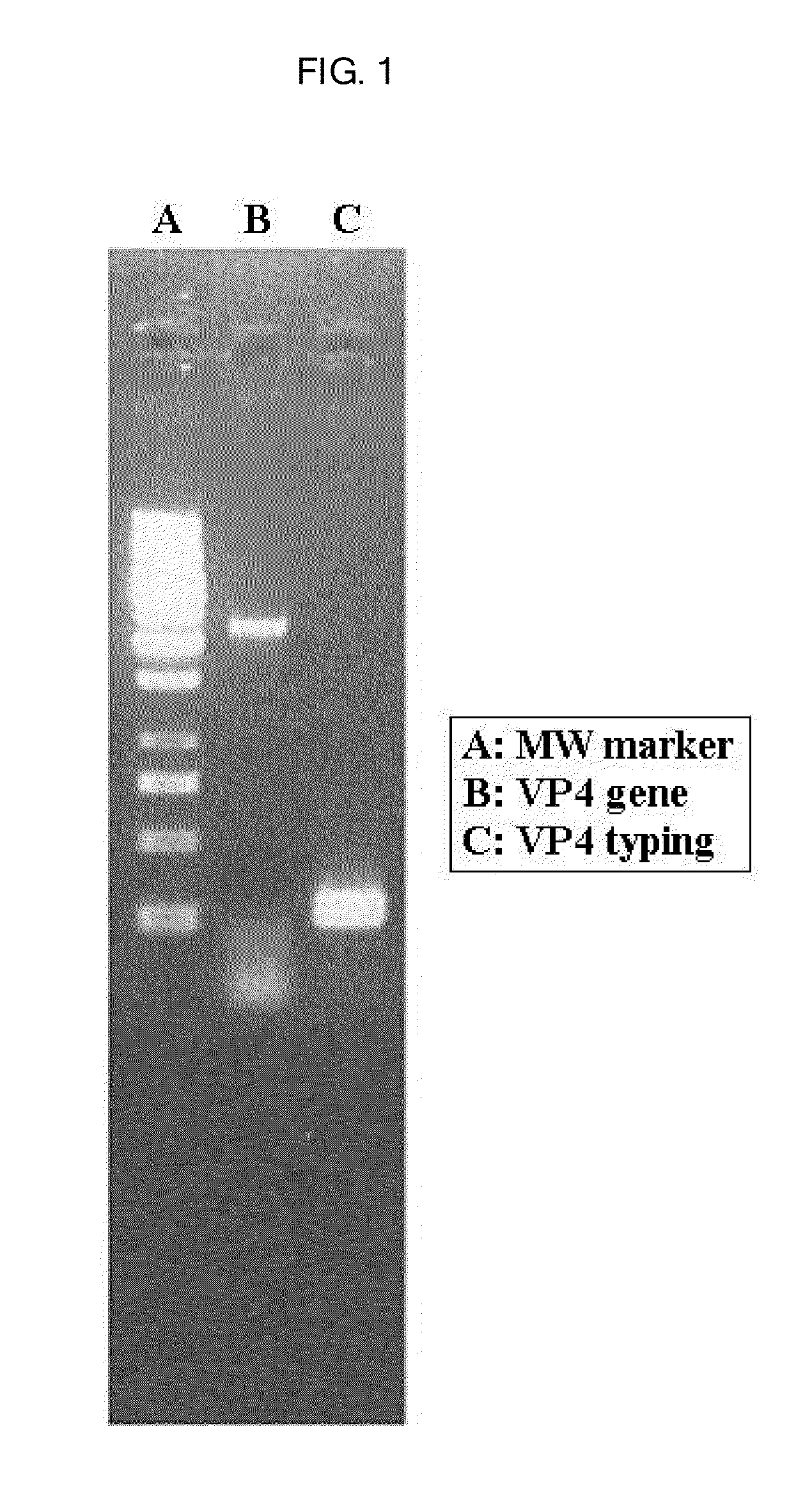
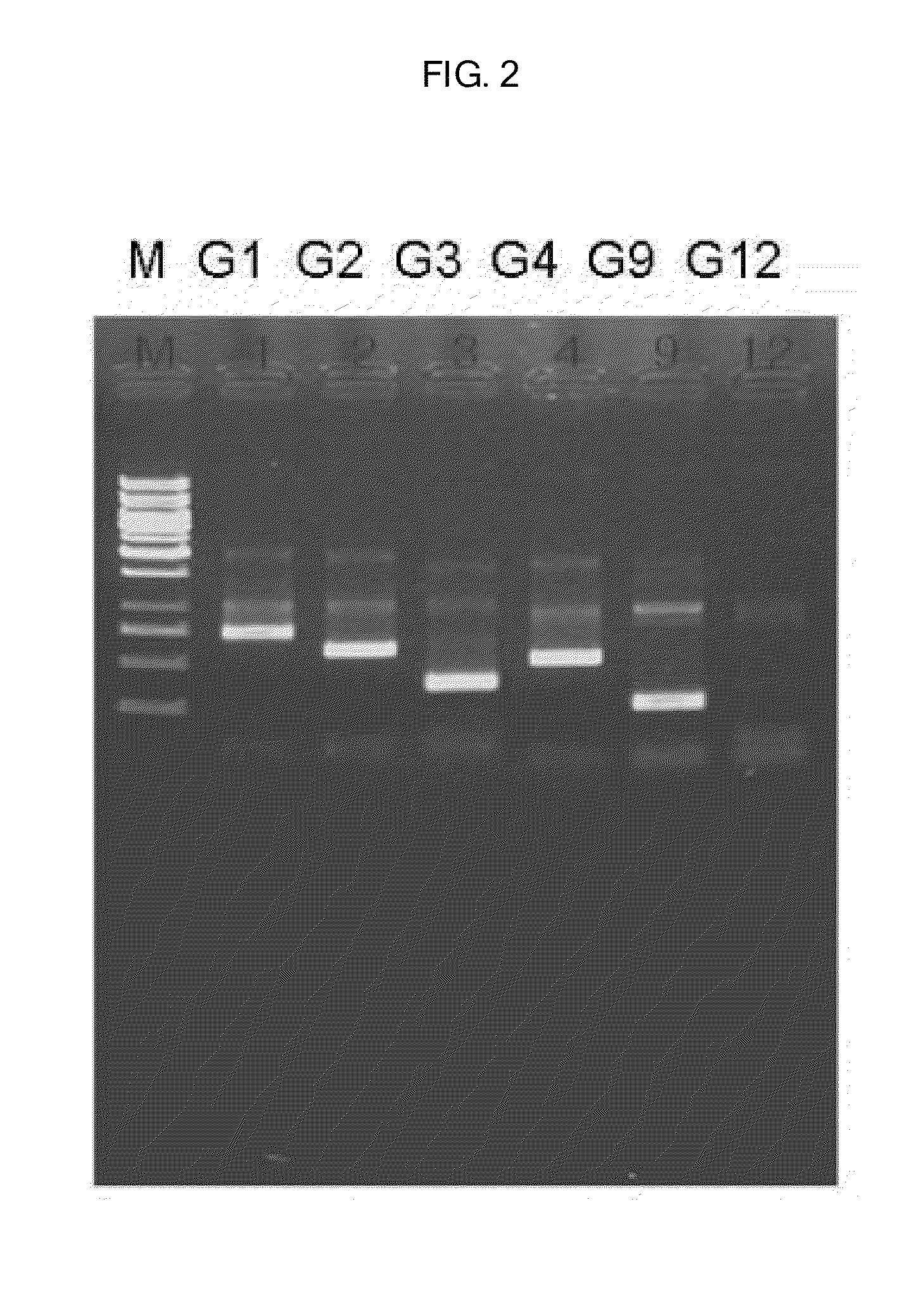
[0047]The result of the seminested multiplex PCR analysis for VP4 gene showed that P genotype of the rotavirus was P[6] as shown in FIG. 1, while those for VP7 gene were two viruses without identifying G serotype thereof as shown in FIG. 2.
Identification of G Serotype of the Two Rotaviruses by Sequencing G7 Gene
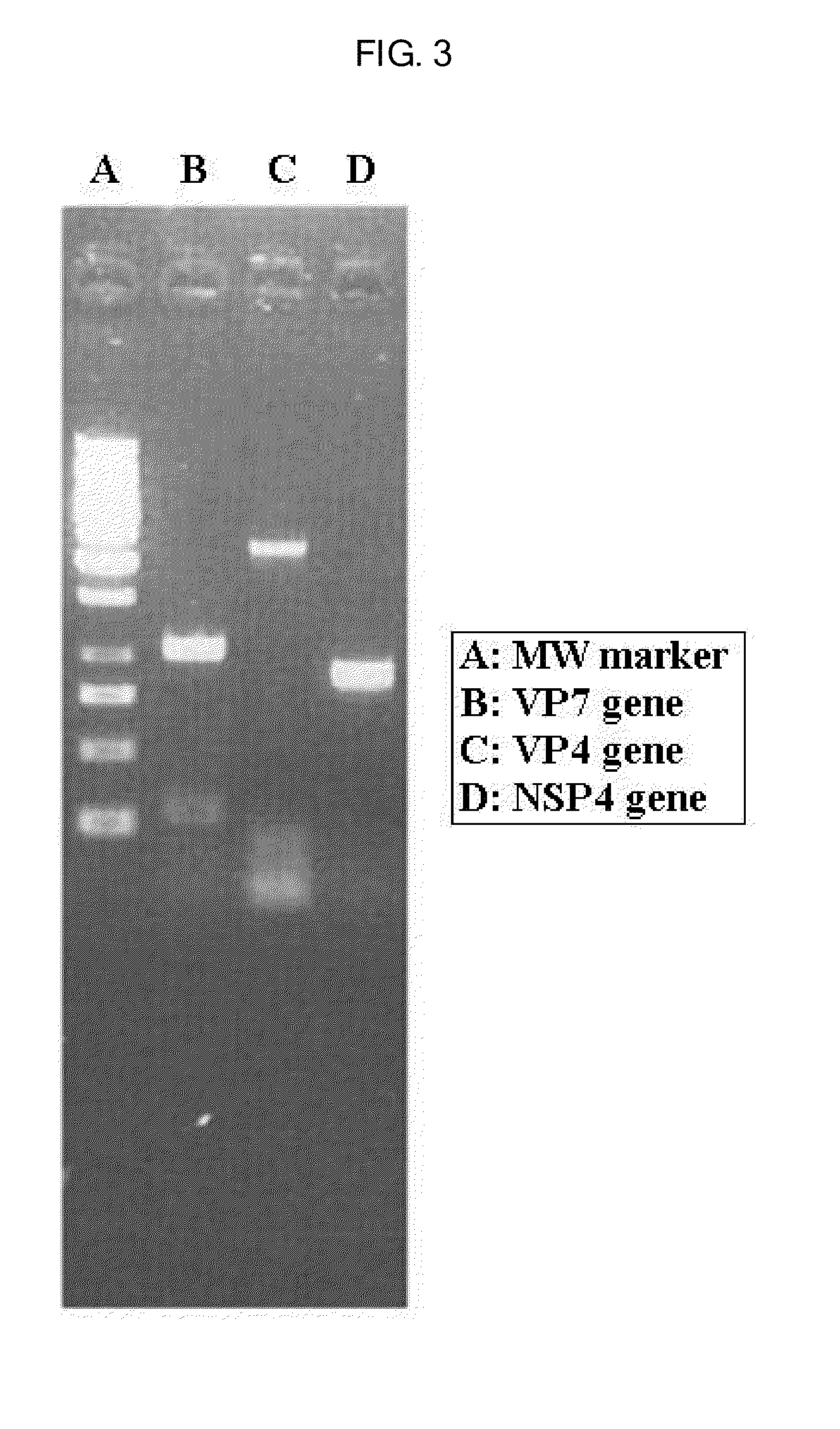
[0048]VP7 gene was amplified by RT-PCR and subjected to gene sequencing to identify G serotype of the two viruses untyped in , as follows. Further, VP4 gene and NSP4 gene were also subjected to RT-PCR and gene sequencing.
[0049]First, the stool specimens obtained in were treated with TRI ...
example 2
Isolation of G12 Human Rotaviruses
[0054]The two rotaviruses, which were determined to have G12 serotype and P[6] genotype in Example 1, were isolated and purified according to the method of Nakagomi et al. (Nakagomi et al., J. Arch. Virol. 127:365-371, 1992). Specifically, the stool specimens were suspended in 5×PBS and filtered through a 0.22 μm filter. Then, the obtained suspensions were infected to MA104 cells (Prof. Linda J Saif, Ohio State University, USA) supplemented with 0.5% pancreatin and cultured in a 5% CO2 incubator until cytopathogenic effect appeared. The proliferated viruses were sub-cultured 2-3 times and subjected to RT-PCR.
[0055]The rotaviruses obtained as above were named as CAU 195 / G12 and CAU 214 / G12, and deposited in a depositary authority as Deposit Accession Nos: KCTC 10988BP and KCTC 10989BP.
[0056]FIG. 5 shows the cytopathogenic effect of the subject rotavirus CAU 195 / G12.
example 3
Study for Genetic Interrelation Between G12 Human Rotaviruses
Study for Genetic Interrelation Between VP7 gene of G12 Human Rotaviruses
[0057]To identify a subclass of CAU 195 / G12 and CAU 214 / G12, VP7 gene sequences thereof as confirmed in were multialigned with those of other G12 rotaviruses, e.g., L26 (Philippine), CP727 and CP1030 (Japan); Se585 (USA); T152 (Thailand); HC91 (Brazil); and RU172 (India), and the result of the multi-alignment was arranged in a similarity matrix using Clustal W (1.7) lo software (Thompson et al., Nucleic Acids Res 22: 4673-4680, 1994). Then, a phylogenetic tree was made based on the similarity matrix using neighbor-joining method (Saitou and Nei, Mol Biol Evo 4: 406-425, 1987).
[0058]As presented in FIG. 6, the nucleotide sequences of V7 genes of CAU 195 / G12 and CAU 214 / G12 showed >90% identity with those of other G12 rotaviruses; 99.5% with each other; 98.6% and 99.2% with Se585 (AJ31174); and 89.5% and 90.3% with L26 (M58290). As also illustrated in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com