Porcine rotavirus delta VP8* subunit recombinant protein and applications thereof
A porcine rotavirus and recombinant protein technology, which is applied in the field of animal molecular biology and genetic engineering, can solve problems such as inability to carry out glycosylation modification, and achieve the effect of improving immune efficacy and good humoral immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
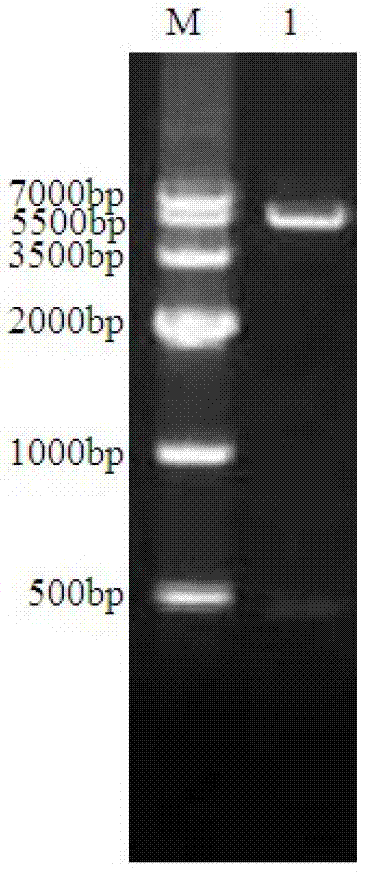
[0053] This example is used to illustrate the construction of the expression vector containing the target fragment.
[0054] The material used is porcine rotavirus OSU strain. Porcine rotavirus OSU strain is a classic strain, which is cultured with primary African green monkey kidney (AGMK) cells. The medium used was EMEM, in which trypsin was added to a final concentration of 0.5 μg / ml, penicillin 100 IU / ml, streptomycin 100 μg / ml, amphotericin B 2.5 μg / ml. The formula of EMEM medium is shown in Table 1:
[0055]
[0056]
[0057] Porcine rotavirus OSU strain RNA was extracted by TRIzol-LS (Invitrogen), and the extracted RNA was reverse-transcribed into cDNA by RT-PCR. The primers used were designed according to the gene sequence of RNA fragment 4 of porcine rotavirus OSU strain VP4 (GenBank accession number: X13190.1). The primers for amplifying ΔVP8* are as follows:
[0058] Upstream primer: 5'-GATC CATATG TTATTAGATGGCCCATACCAACC-3' (SEQ ID No.3), the underlined ...
Embodiment 2
[0064] This example is used to illustrate the expression of porcine rotavirus subunit recombinant protein.
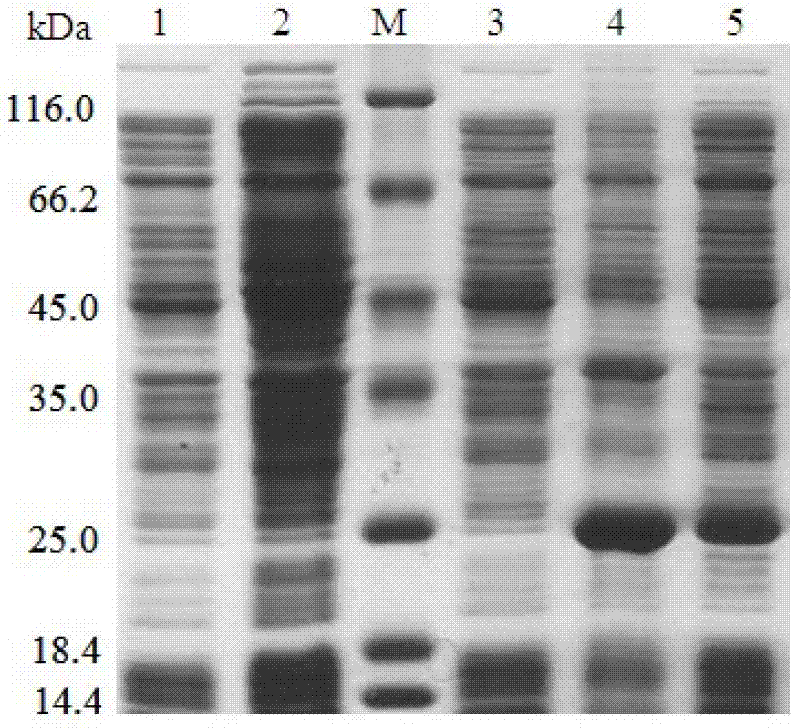
[0065] Single colonies of positive recombinant bacteria and empty vector control bacteria were picked respectively, inoculated in 3 mL of LB liquid medium containing 30 μg / mL of kanamycin, and cultured with shaking at 37°C for 12 to 16 hours. Then transfer the bacterial liquid to 10 mL of fresh LB liquid medium (containing 30 μg / mL kanamycin) at a ratio of 1%, shake culture at 37°C and 200 rpm until OD600≈0.6, and take out 1 mL of bacterial liquid as an induction For the former control, add IPTG to the remaining bacterial solution to a final concentration of 0.5mM, lower the temperature to 22°C, and induce expression overnight. Take 1mL post-induction bacterial solution and 1mL pre-induction bacterial solution respectively, collect the bacterial cells by centrifugation, resuspend the bacterial cells in 0.01M PBS (pH7.4), and then ultrasonically disrupt them in an ice ba...
Embodiment 3
[0067] This example is used to illustrate the purification of recombinant proteins.
[0068] The cell wall of E. coli cells was disrupted with BugBuster Master Mix (Novagen) containing protease inhibitor cocktail (Roche), and the soluble product was collected and stored at -80°C for future use. Each protein was purified by ProBond nickel-NTA agar affinity chromatography (Invitrogen) according to the manufacturer's protocol. After the cellular protein was washed away with wash buffer containing different concentrations of imidazole (20-100mM), the recombinant protein was eluted with 250mM imidazole. The purity of the recombinant protein was analyzed by SDS-PAGE, and imidazole in the solution was removed with a centrifugal filter device (Millipore). According to the BCA Quantification Kit (Thermo) manufacturer's instructions, the BCA Quantification Kit was used to determine the concentration of the purified recombinant protein. The purified recombinant protein was stored at -8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com