Method for the determination of the formation of endothelins for medical diagnostic purposes, and antibodies and kits for carrying out such a method
a technology of endothelin and determination method, which is applied in the field of determination of the formation of endothelins for medical diagnostic purposes, and antibodies and kits for carrying out such a method. it can solve the problems of possible further processing and stability, and nothing has been disclosed to da
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
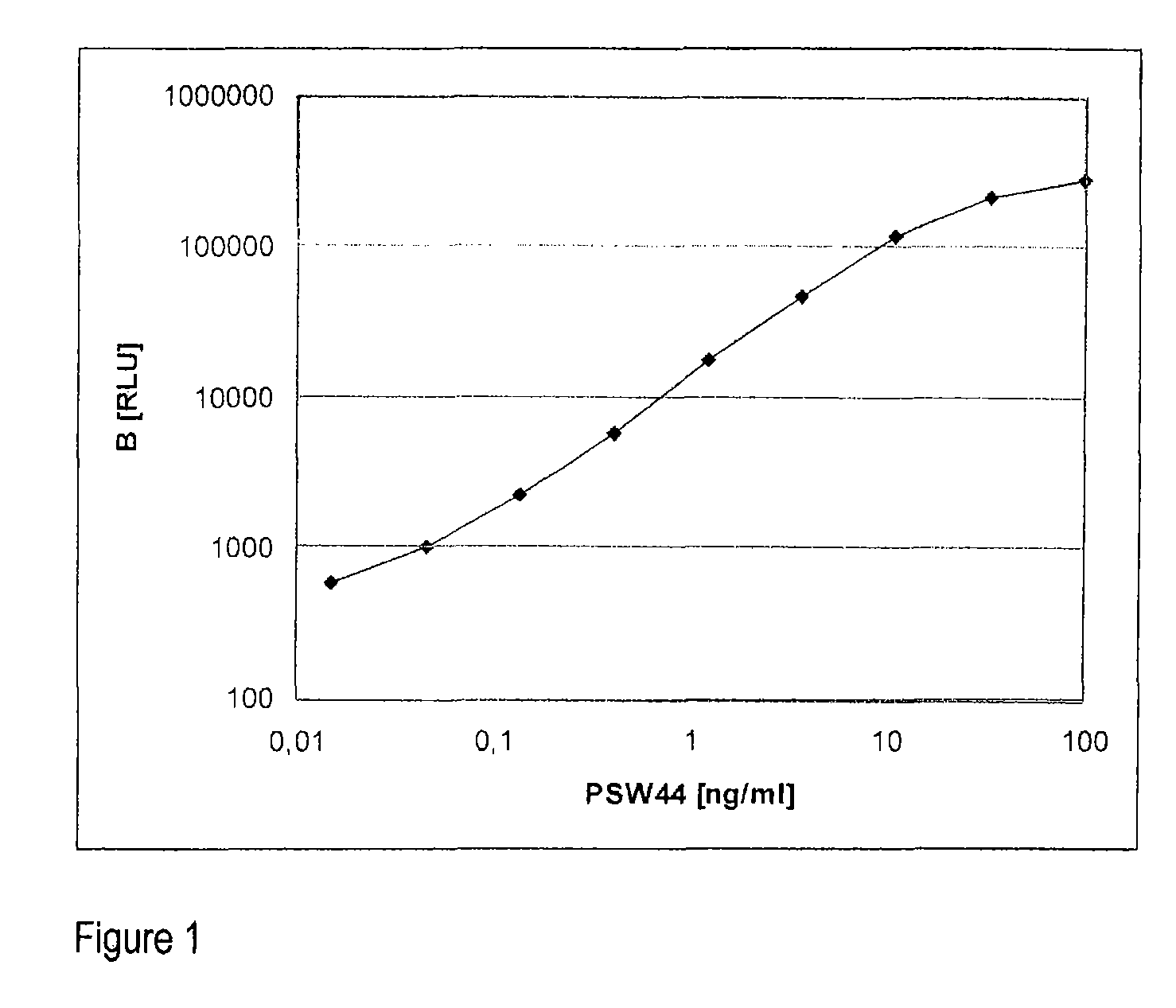
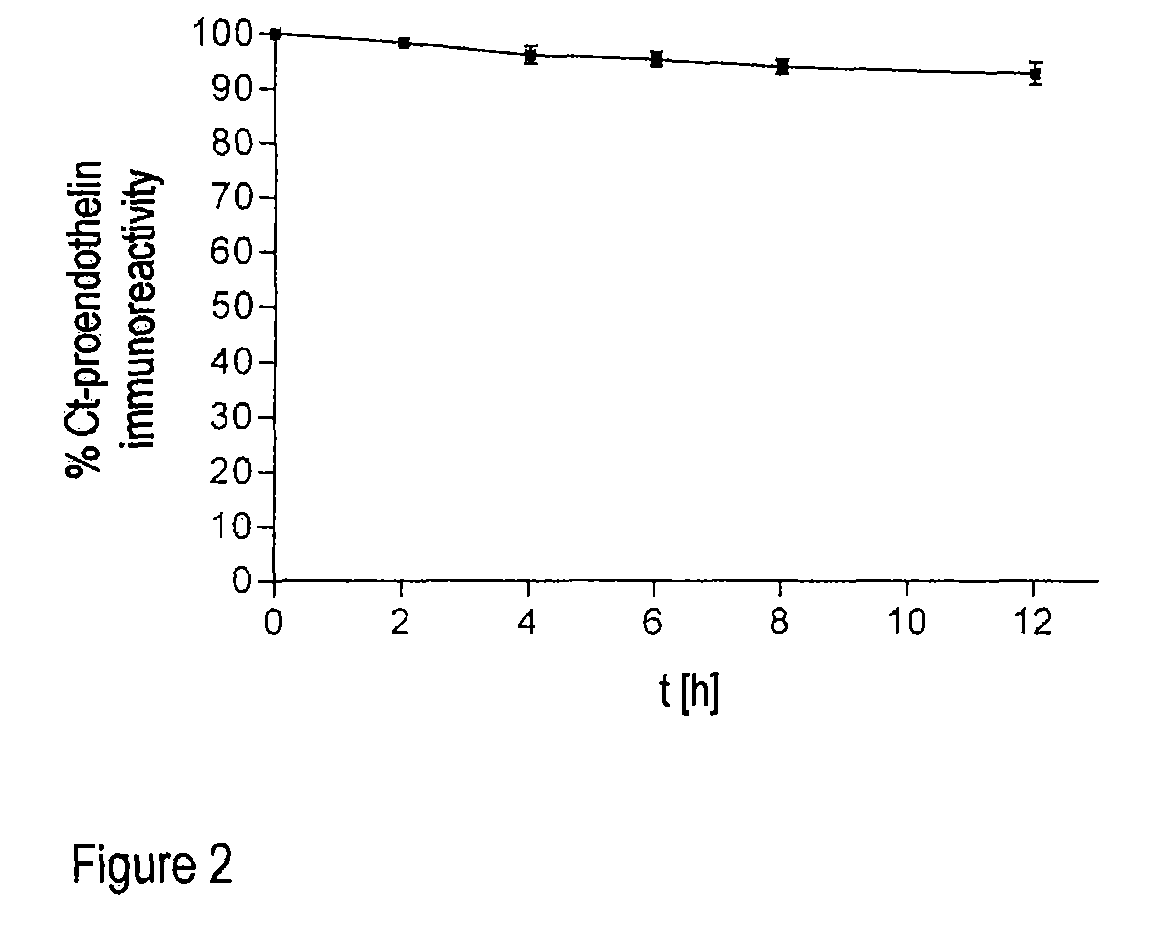
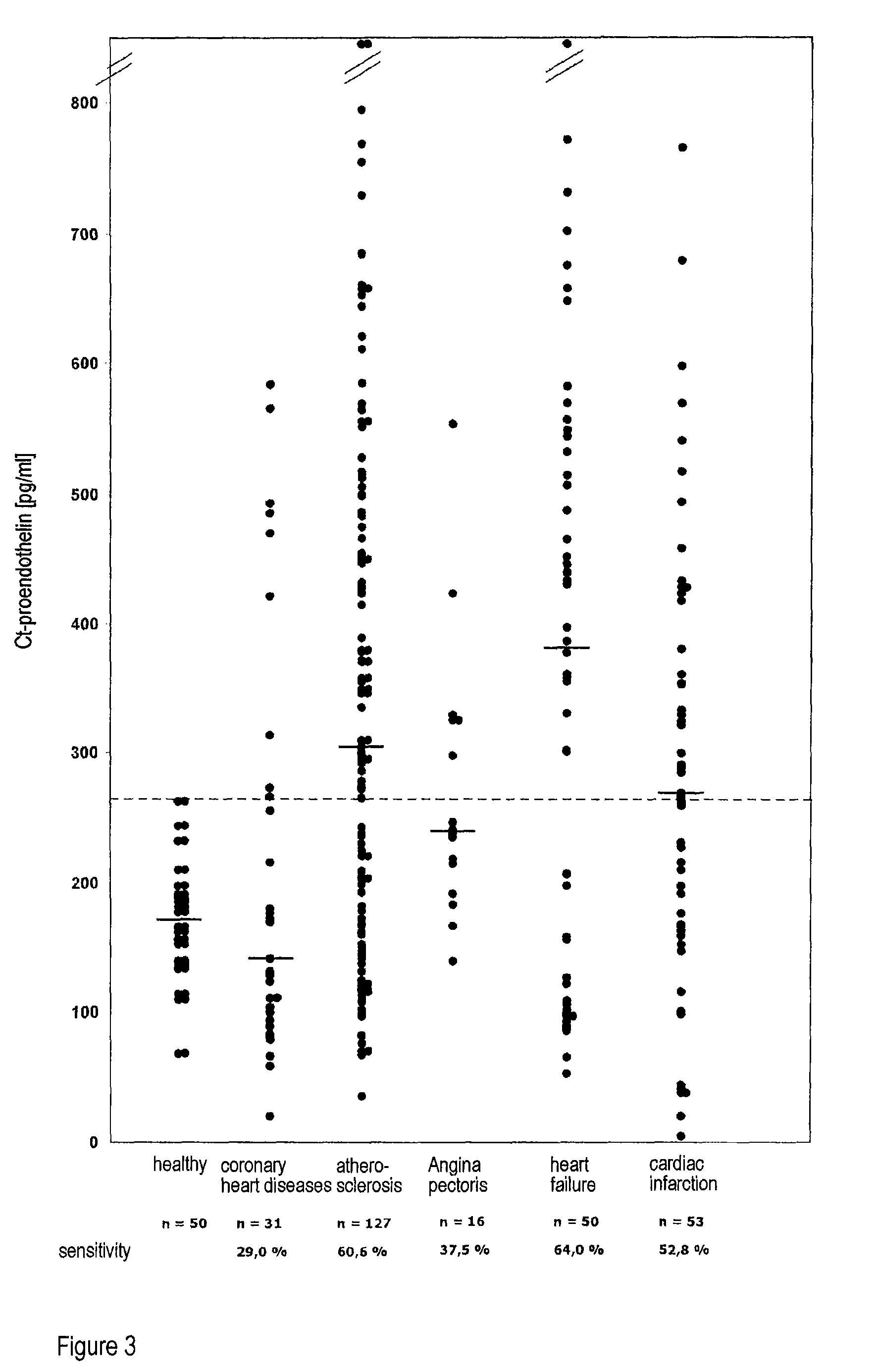
[0029]The method according to the invention relates in its most general aspect to the determination of a relatively long-lived peptide fragment of proendothelin-1 which does not contain the amino acid sequences of endothelin-1 or its precursor big endothelin, in whole blood, plasma or serum samples, i.e. in the circulation of patients, for the indirect determination of the formation of endothelins, in particular of endothelin-1, in serious diseases. According to a preferred embodiment, the peptide fragment determined is a C-terminal fragment to which two antibodies bind which bind to peptides having amino acid sequences which correspond to the positions 168-181 and 200-212 of preproendothelin-1.
[0030]For the practical implementation of the invention, noncompetitive sandwich assays, for example of the type as used for the more far-reaching detailed investigations and described more exactly below, are particularly preferably provided.
[0031]Compared with competitive immunoassays, nonco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| residence time | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com