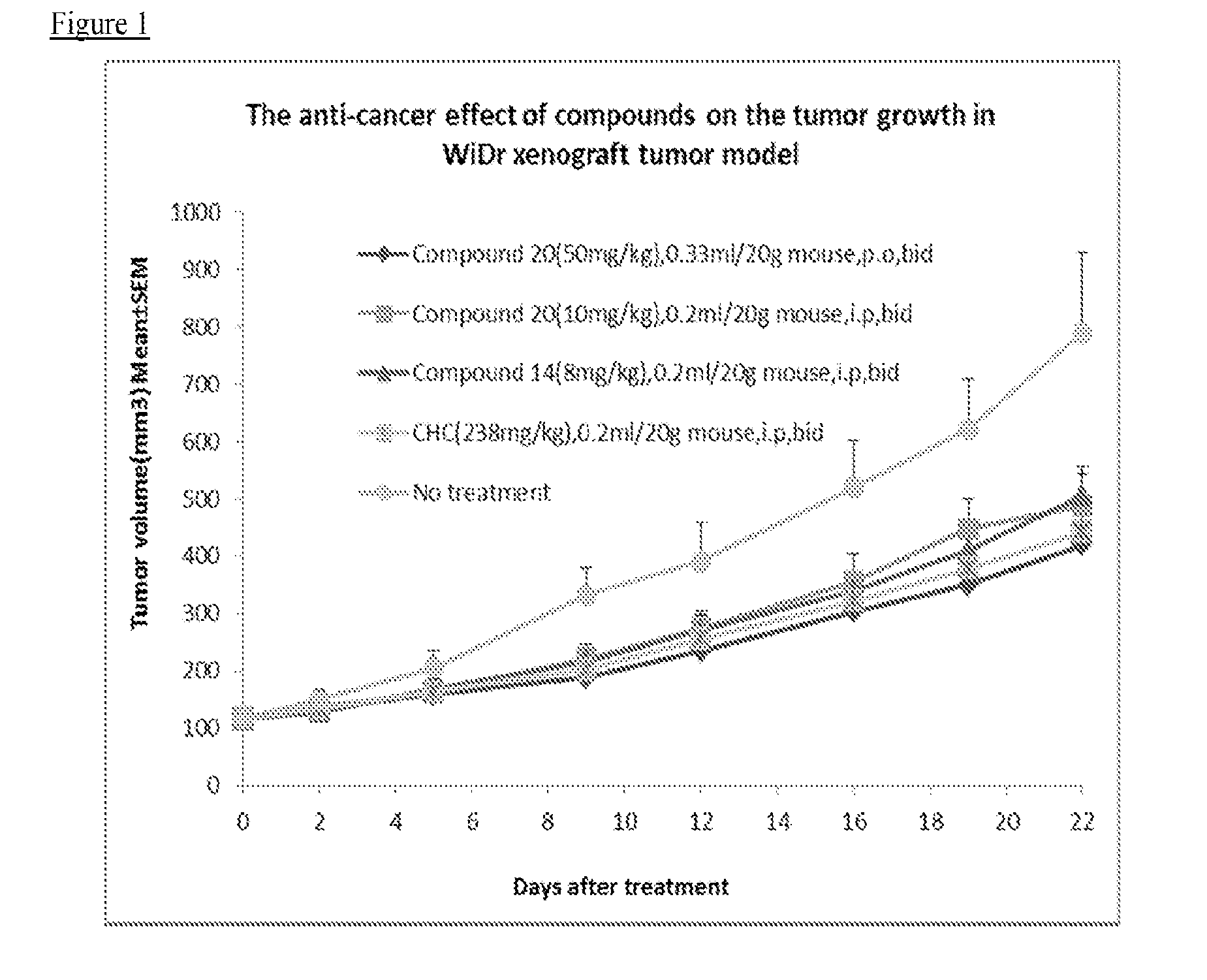
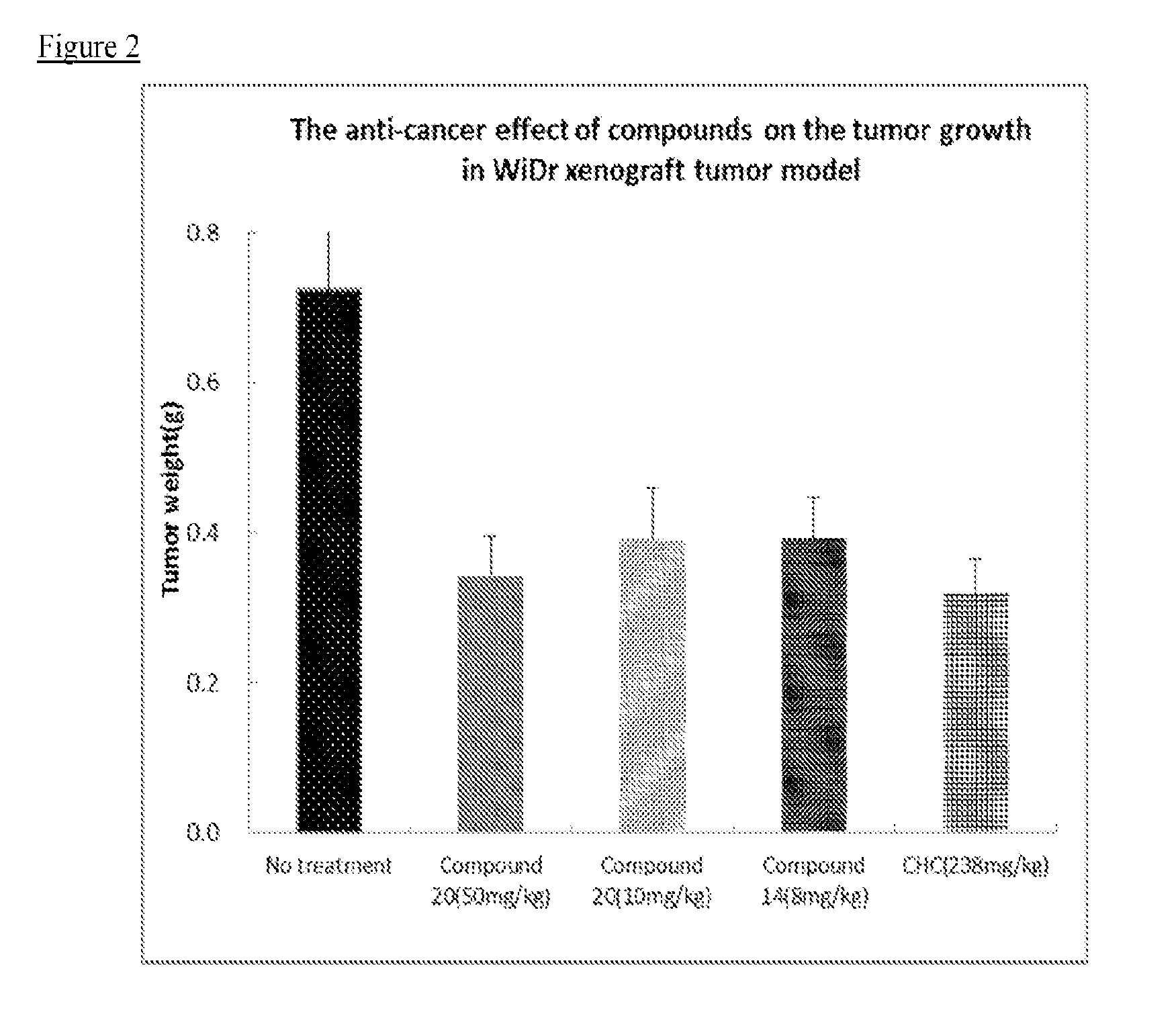
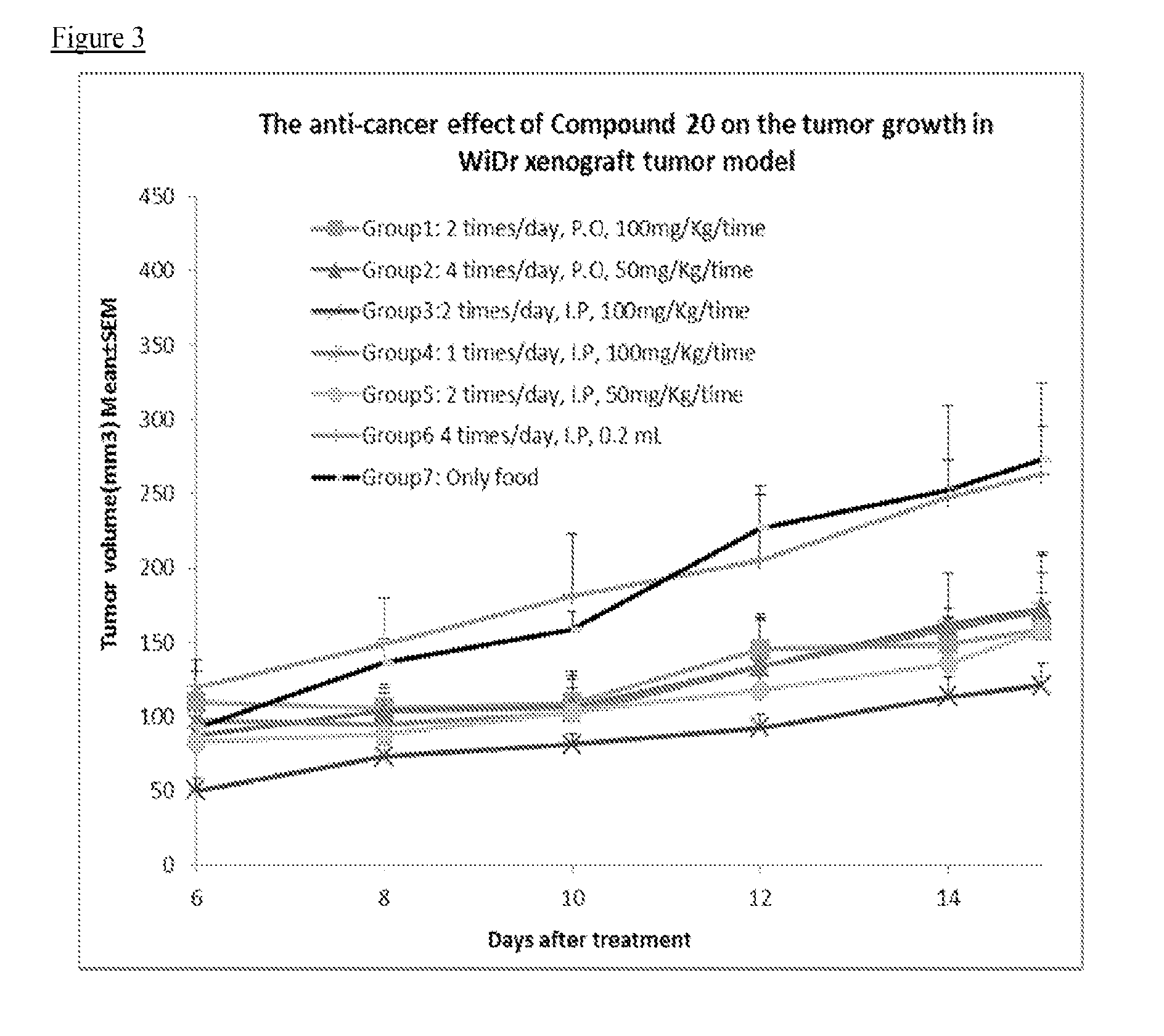
Therapeutic compounds
a technology of compounds and compounds, applied in the field of therapeutic compounds, can solve the problems of increased cancer risk, metastasis, relapse, patient mortality,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compounds 4-12 (Table I)
[0195]
[0196]To a solution of the substituted benzaldehyde (10 mmol) in 20 ml acetonitrile or ethanol, was added cyanoacetic acid (15 mmol) and piperidine (10 mmol) and refluxed for 3-8 h at 80° C. Upon the completion of the reaction, the above solution was poured into a mixture of 3M HCl (10 mL) in ice. The solution was stirred for 10 to 15 minutes and the solid was filtered using Buchner funnel. The solid was washed with water and acetonitrile. The pure compound was obtained upon recrystallization.
[0197]
TABLE 2ElementalElementalCompoundCalculatedObservedYieldNumberCompoundC %H %N %C %H %N %(%) 471.978.059.3371.838.389.3565 573.148.598.5372.618.378.1670 674.129.057.8674.189.997.7468 771.626.0110.4470.986.6611.3660 872.724.5810.670.624.710.2862 971.978.059.3371.488.499.23611078.245.477.6077.305.337.88721169.415.8211.5668.755.5011.20681270.296.2910.9370.516.4511.0167
example 2
Synthesis of Compounds 13-21 (Table 3)
[0198]
[0199]To a solution of the substituted o-methoxy-benzaldehyde (10 mmol) in 20 ml acetonitrile or ethanol was added cyanoacetic acid (15 mmol) and piperidine (10 mmol) and refluxed for 10-20 h at 80° C. Upon the completion of the reaction, the above solution was poured into a mixture of 3 M HCl (10 mL) in ice. The solution was stirred for 10 to 15 minutes and the solid was filtered using Buchner funnel. The solid was washed with water and acetonitrile and the pure compound was obtained upon recrystallization.
[0200]
TABLE 3ElementalElementalCompoundCalculatedObservedYieldNumberCompoundC %H %N %C %H %N %(%)1367.537.339.2667.117.359.14621469.067.938.4870.017.668.56601570.368.447.8170.518.607.92741668.446.089.3968.596.129.48701769.384.799.5269.894.999.78661869.067.938.4868.957.578.66581975.365.577.0375.795.867.34622074.584.907.5674.124.477.82662166.165.9210.2966.365.8610.3168
example 3
Synthesis of Compounds 22-29 (Table 4)
[0201]
[0202]To a solution of substituted o-hydroxy-benzaldehyde (10 mmol) in 20 ml ethanol, was added diethyl malonate (20 mmol) and piperidine (13 mmol) and refluxed for 8-12 h at 80° C. Upon the completion of the reaction, the above solution was evaporated and further refluxed in 10 ml of 10% NaOH solution. The reaction was quenched with 3M HCl and worked up with ethyl acetate. The compound was purified by column chromatography (eluted with 100% ethyl acetate) and recrystallized.
[0203]
TABLE 4ElementalElementalCompoundCalculatedObservedYieldNumberCompoundC %H %N %C %H %N %(%)2266.426.624.8466.546.764.91502367.365.304.9167.385.194.98642468.323.944.9868.393.974.86532574.794.973.6374.874.563.84582664.865.055.4064.214.875.12592768.414.073.3268.664.343.38552863.453.773.0863.553.842.97572965.964.052.9665.873.873.0855
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap