Method, system and apparatus for improved micromanipulation and storage
a micromanipulation and storage technology, applied in the field of manipulation and handling of biological materials, can solve the problems of biological material being subject to potential ice crystal formation, cryopreservation process, high degree of trauma to biological material in question, etc., to increase the cryopreservation quality, and reduce the osmotic shock to the embryo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
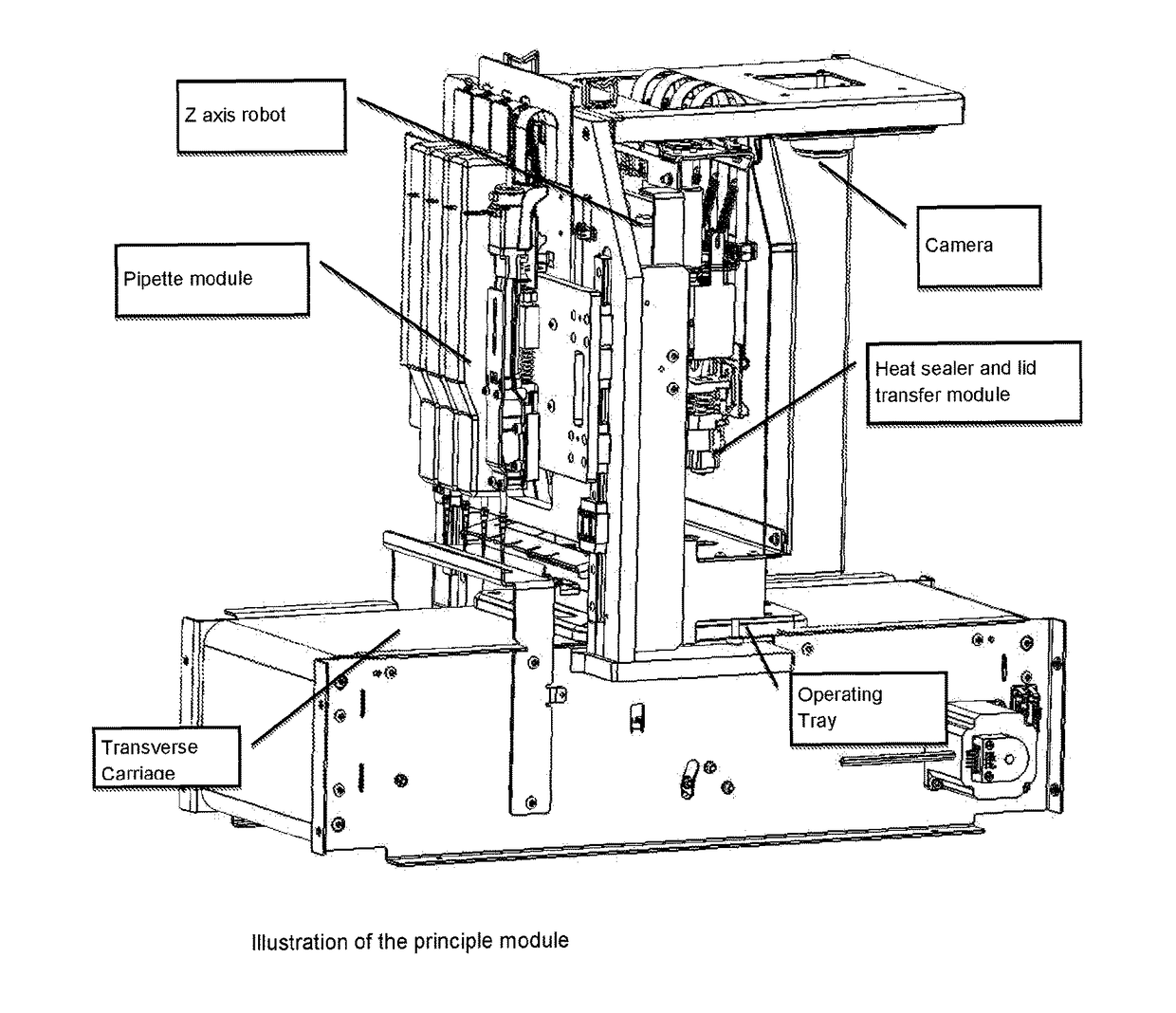
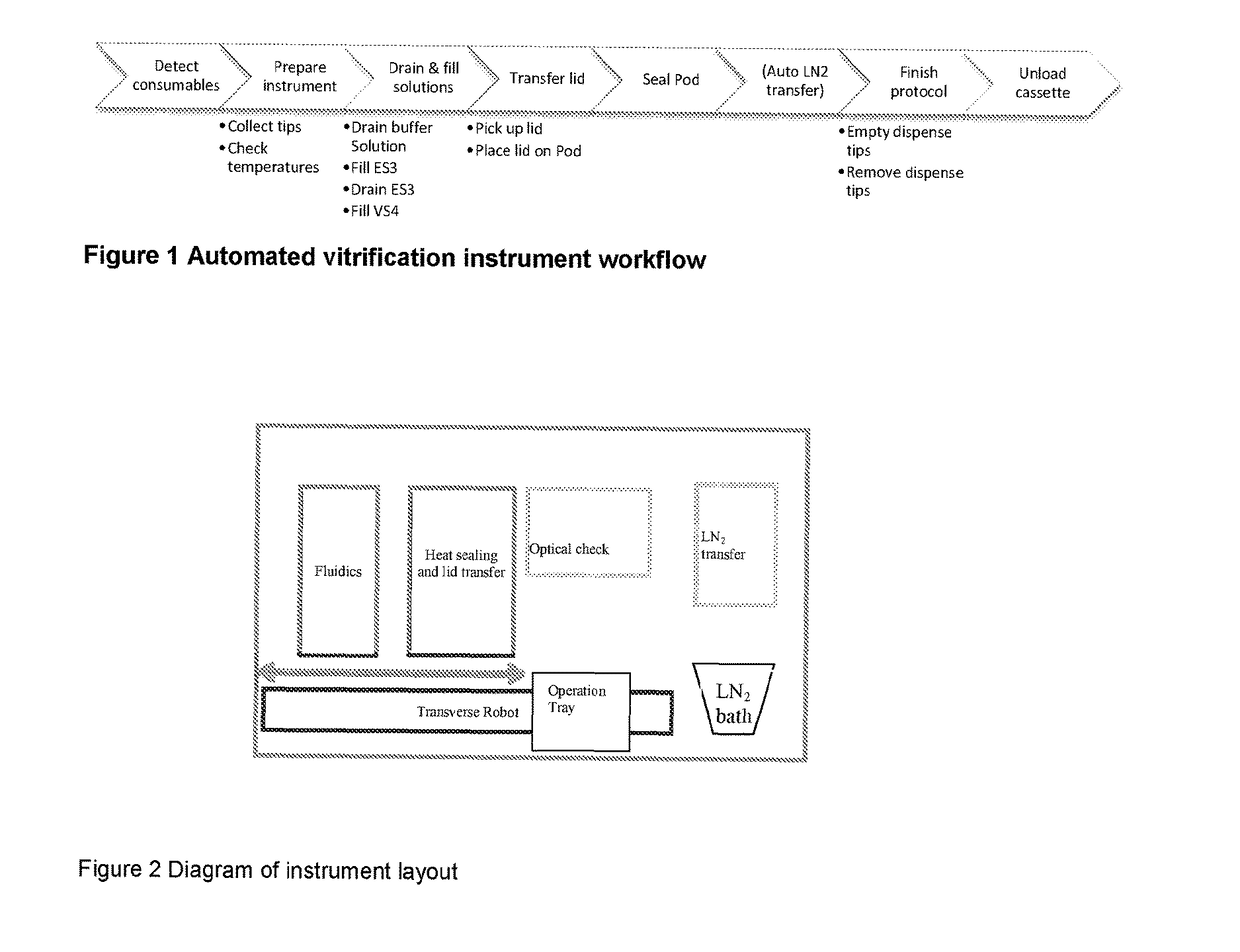
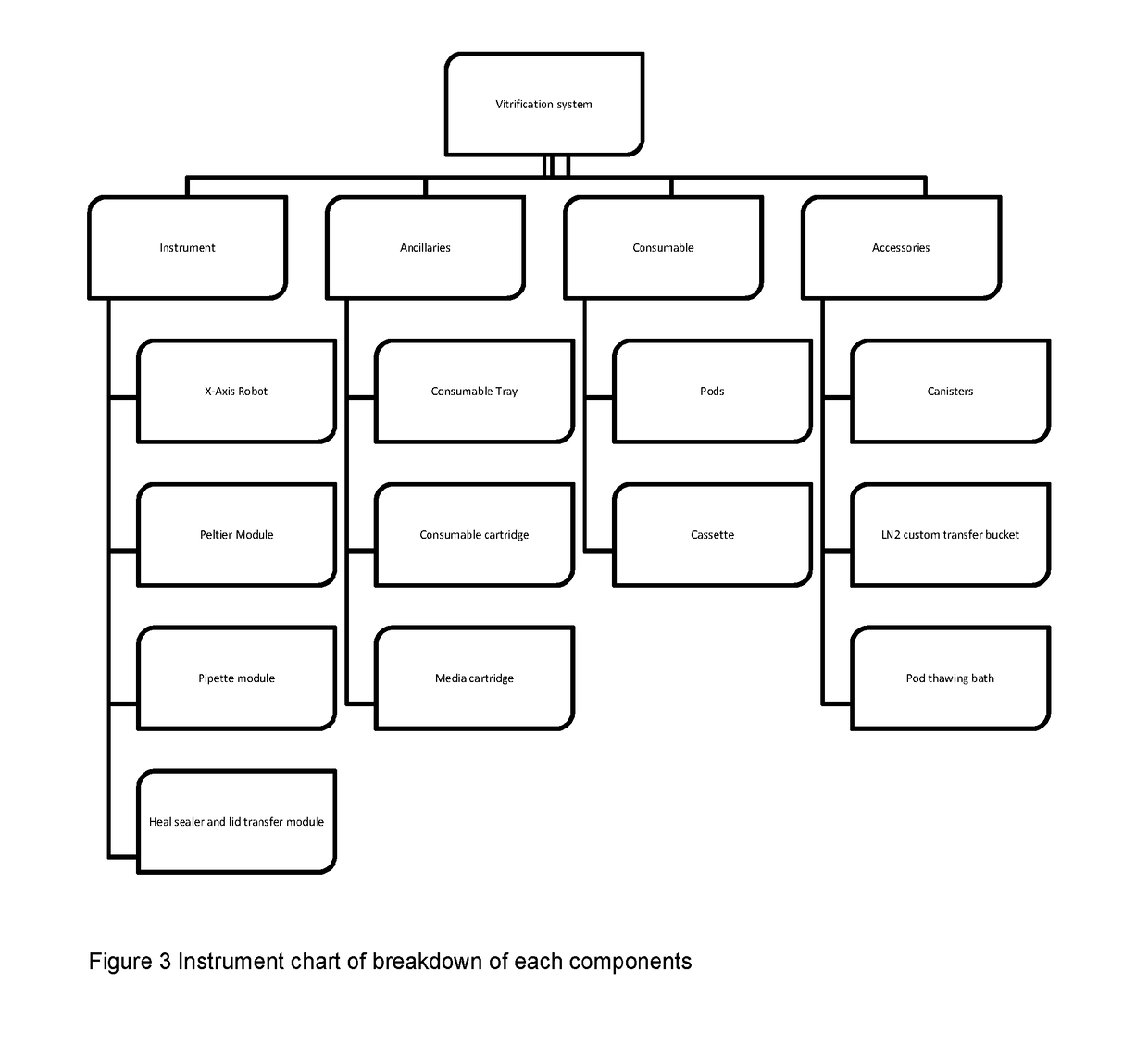
[0112]For the purposes of this description, the following definitions apply. The term “embryo” in this document refers to an embryo, mammalian or non-mammalian, which includes but is not limited to a human embryo at stages commonly occurring during the period when the embryos can be kept in in vitro conditions in the laboratory, commonly days 1 to 6 from oocyte retrieval. The term “embryo”, implies also the “oocyte”, unless otherwise specified, where an oocyte is taken to be an unfertilised metaphase II stage 1-cell egg before fertilization or an immature GV stage oocyte before final oocyte maturation. “Solution” relates to fluid used for the purpose of cryopreservation of an embryo. The term “consumable” refers to disposable low cost devices designed for accommodating and handling the embryo or oocyte for introduction and preparation for vitrification as handled by a user or technician and interfaces to laboratory instrumentation. A “cassette” may be the holder / platform in which mu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com