Methods and compositions for isolating exosomes
a technology of exosomes and compositions, applied in the field of biotechnology, can solve the problems of time-consuming, cumbersome and/or costly, limited by the amount of material that can be processed, and achieve the effects of rapid, efficient and cost-effectiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
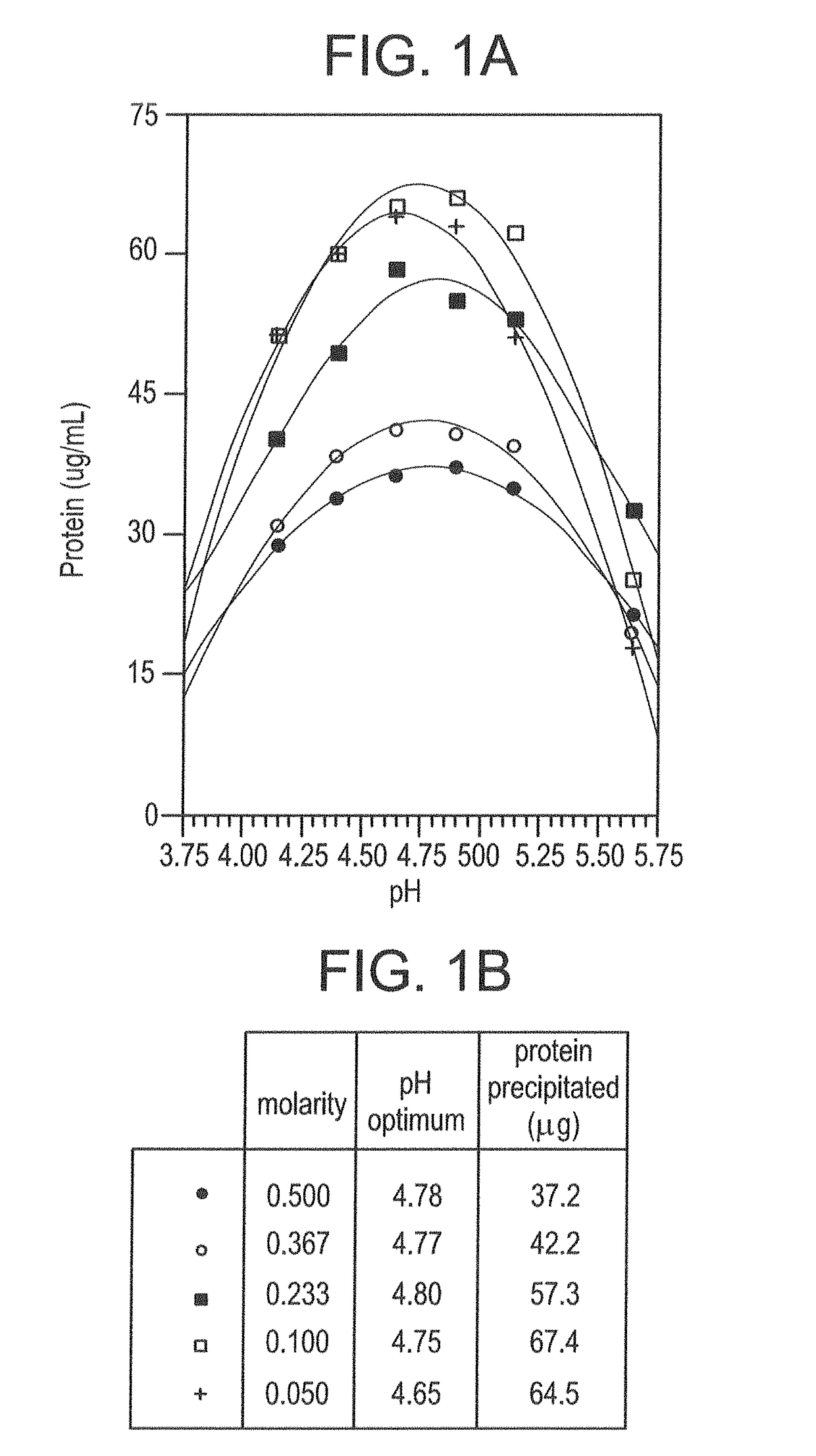
Isolating Tumor-Derived Exosomes Using Acetate Buffers
[0149]The present example shows advantageous methods for isolating extracellular microvesicles such as exosomes, particularly tumor-derived exosomes, using acetate buffers.
A. Materials and Methods
[0150]The following Materials and Methods are relevant to the results reported in Example I, Example II and Example III.
[0151]1. Tissue Culture
[0152]K1735P murine melanoma (tumor) cells (provided by I. J. Fidler, M. D. Anderson Cancer Center, Houston, Tex., but anyway widely available) were cultured in minimal essential media (MEM) supplemented with L-glutamine (2 mM), Na pyruvate (1 mM), penicillin (100 U / mL), streptomycin (100 μg / mL), nonessential amino acids and fetal bovine serum (10%). Cells (about 25×106 in 15 mL media) were seeded into the lower chamber of CELLine AD 1000 flasks (Integra Biosciences AG) that contained 250 mL media in the upper chamber (Mitchell et al., 2008). Conditioned media (about 15 mL) was collected from the ...
example ii
Further Characterization of Tumor Exosome Isolation
[0169]This example further characterizes the isolation methodology for disease-related extracellular microvesicles such as exosomes, and highlights the importance of acetate buffers in the technique with reference to tumor exosomes.
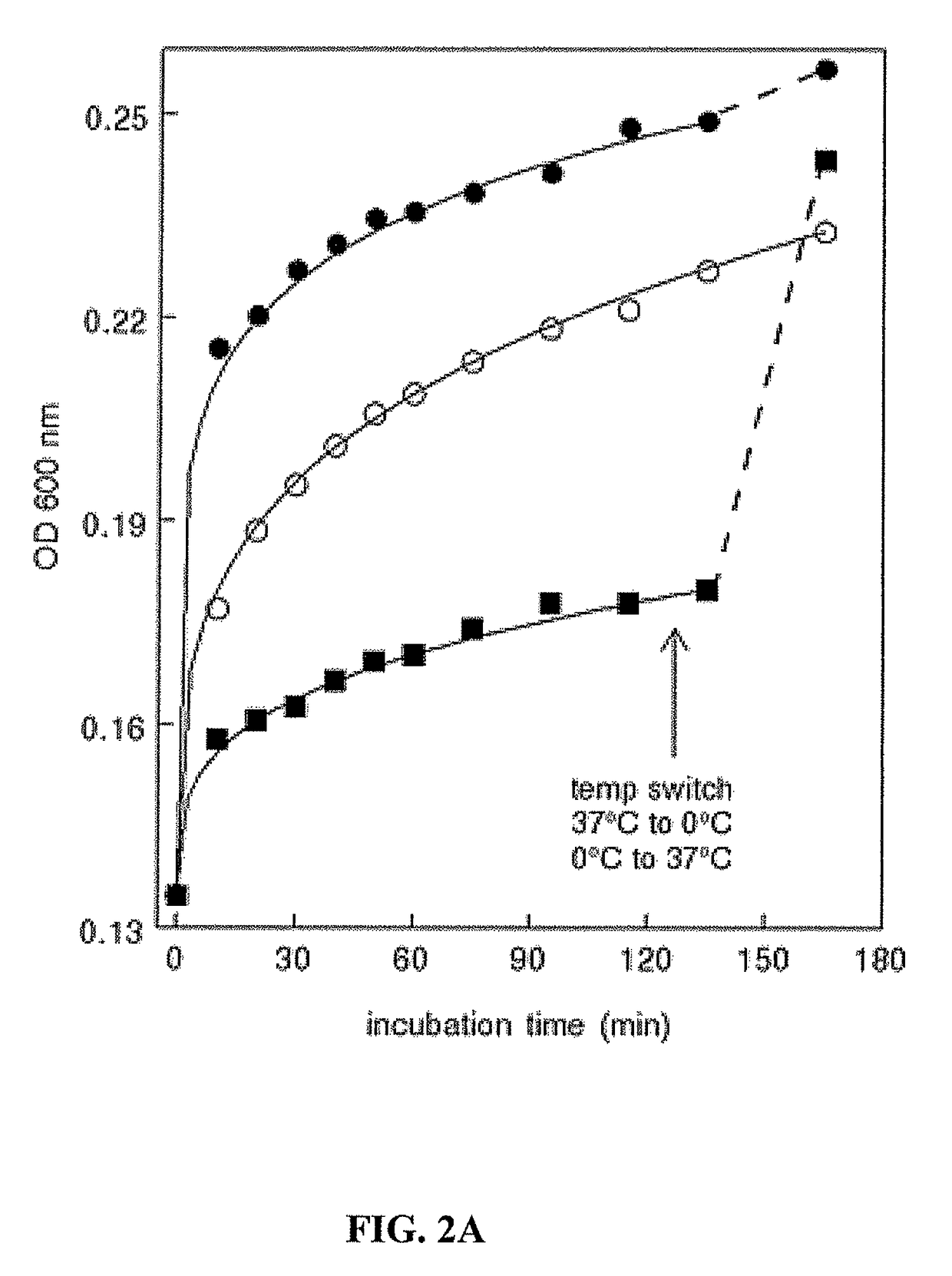
A. Temperature Independence
[0170]The present study shows that tumor exosome precipitation is essentially temperature-independent. However, analyzing the effect of temperature showed that the development of turbidity was temperature-dependent.
[0171]An immediate temperature-dependent increase in turbidity occurred upon the addition of acetate, which then began to level off. Continued incubation showed a modest, about 2-fold increase in rate between 0° C. and 20° C. However, no significant difference in rate was observed upon increasing the temperature from 20° C. to 37° C. (FIG. 2A). Interestingly, once the reaction plateaued at 0° C., increasing the temperature to 37° C. resulted in an immediate increase i...
example iii
Equivalent Yield of Morphologically Verified Tumor Exosomes
[0179]The present example shows that the yield of disease-related extracellular microvesicles such as exosomes isolated using acetate buffers is equivalent to that from the typical ultracentrifugation method, even though the acetate method is easier and quicker. The acetate-purified exosomes, as exemplified by tumor exosomes, are also shown to be morphologically indistinguishable from those prepared by traditional ultracentrifugation and to be antigenically intact.
A. Equivalent Yield
[0180]The relative yield of tumor exosomes obtained with acetate isolation and with conventional 100,000 g ultracentrifugation was quantified by assessing alix and PS in both populations. Flow cytometry analysis of alix with Cy3-labeled alix antibodies (FIG. 5A) and PS with FITC-labeled annexin 5 (FIG. 5B) suggested that both methods yielded similar amounts of exosomes.
[0181]In directly comparing the yield of protein obtained with acetate isolati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com