Materials and methods relating to packaging cell lines
a technology of packaging cell lines and materials, applied in the direction of viruses/bacteriophages, active genetic ingredients, genetic material ingredients, etc., can solve the problems of stringent safety characterisation and scale up required for good manufacturing practice, compromising the safety of virus batches produced, etc., and achieves stable transgene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
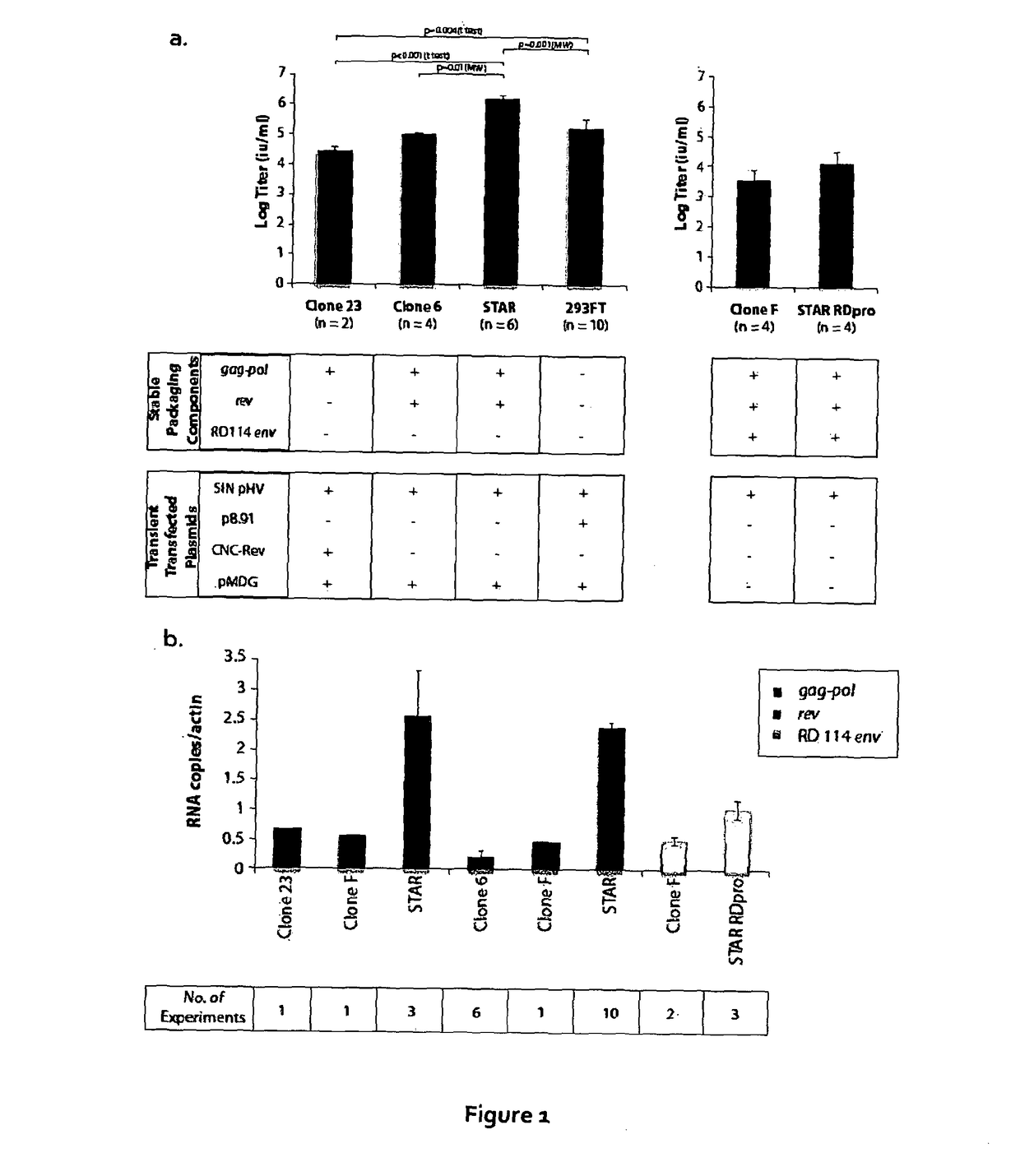
[0159]293FT cells were transduced with a gammaretroviral vector encoding HIV gag-pol. The gammaretroviral vector was similar to that used in STAR cells apart from the LTR, where an enhancer deletion made the vector self-inactivating (SIN). A clone (clone 23) with a single vector integration site, producing the highest level of gag-pol as measured by p24 ELISA, was then transfected with a plasmid, expressing rev under the control of the CMV promoter and the hygromycin resistance gene under the herpes simplex virus thymidine kinase (TK) promoter, and clones with stable integrations were selected using hygromycin. Rev expression was checked by western blot and a clone (clone 6) was chosen for the next step where a plasmid containing the RD114 envelope with an MLV cytoplasmic tail (RD+) was transfected and clones with stable integrations selected using phleomycin. Expression of RD+ was assessed by western blot and one clone (clone F) was chosen for the next stage.
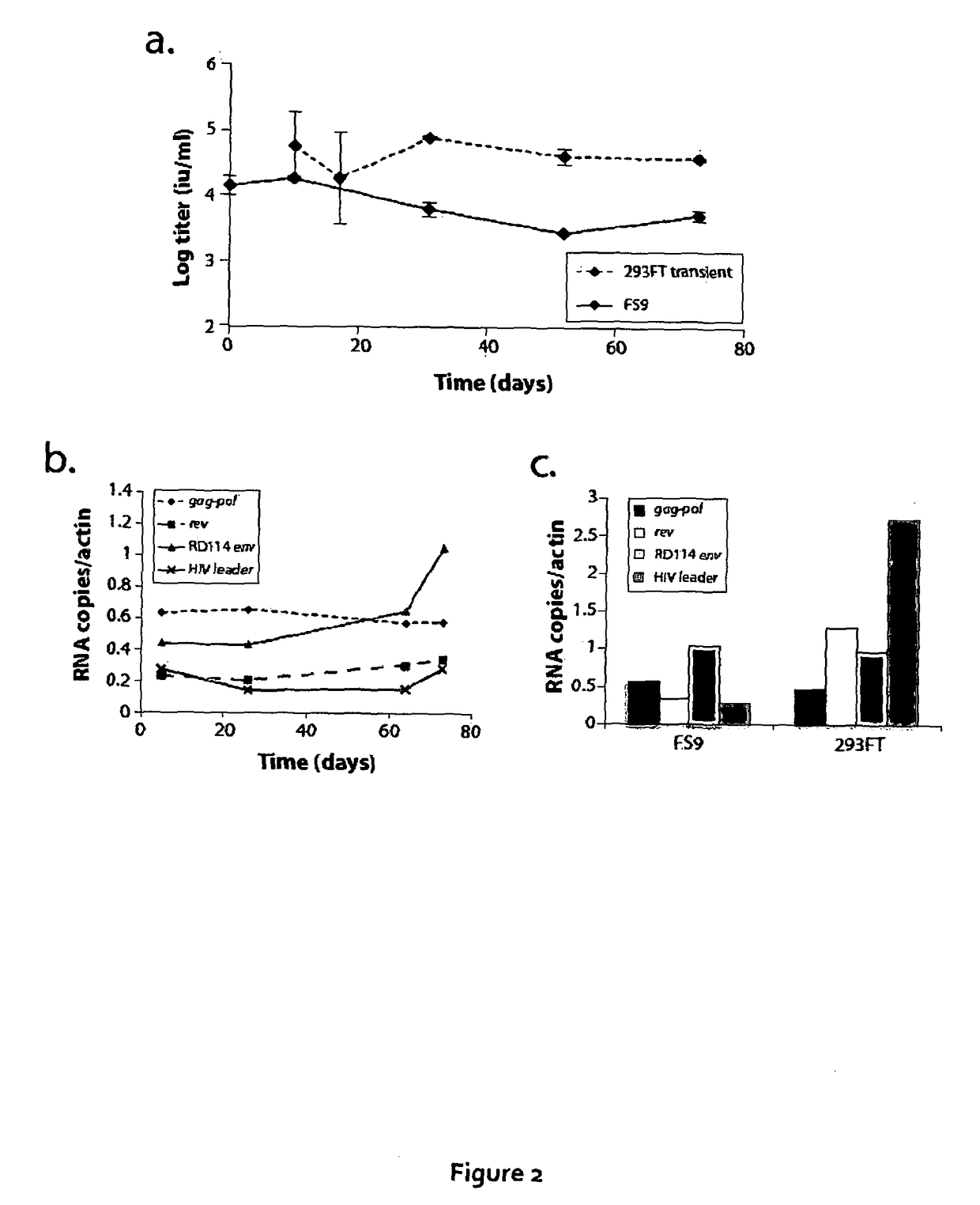
[0160]The inventors the...
example 2
[0172]The inventors then attempted to make a lentiviral packaging cell line from 293FT cells. This differed from the first attempt described in Example 1 in the codon optimised HIV gag-pol that was used, as well as the method of HIV gag-pol expression.
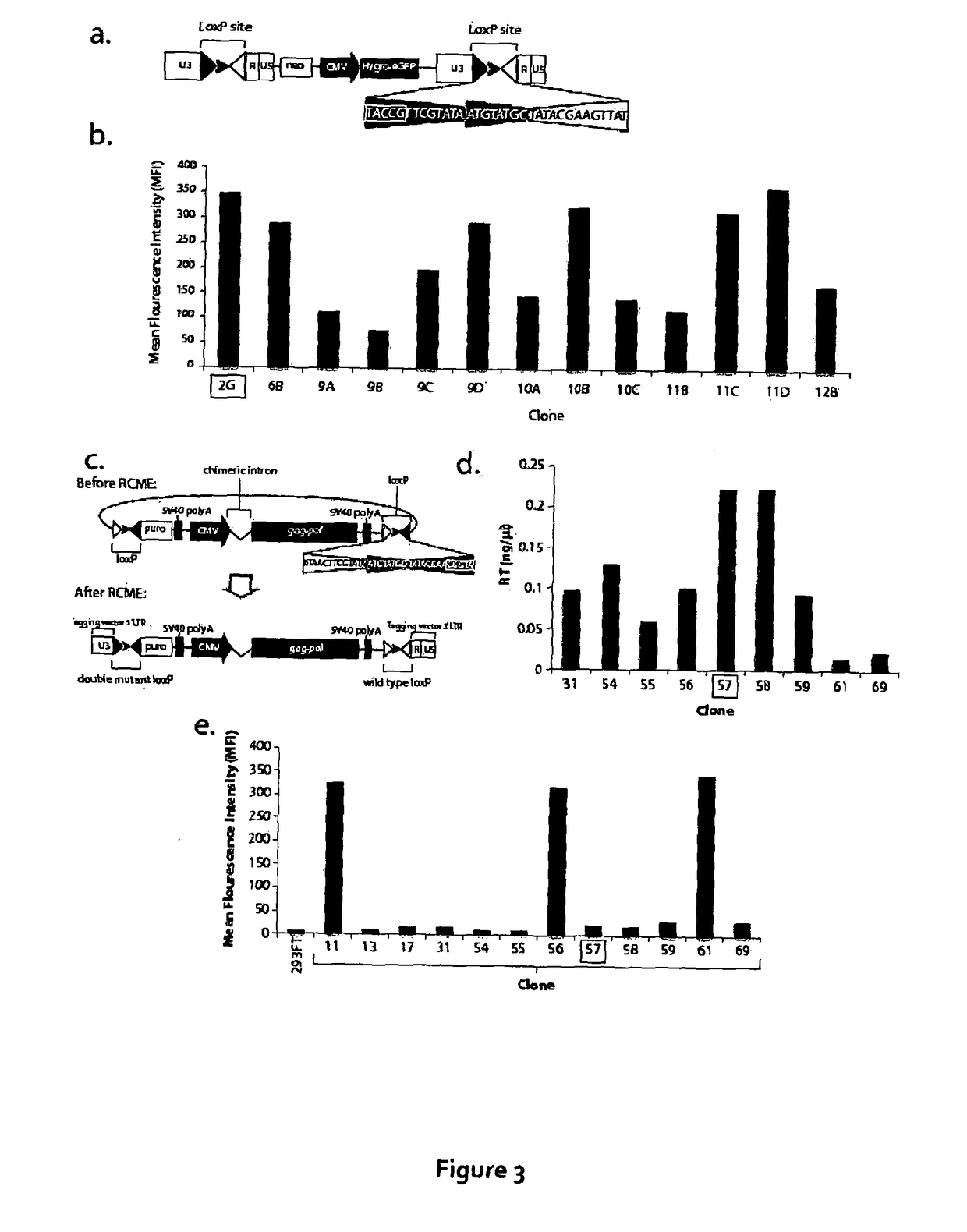
[0173]Recombinase-mediated cassette exchange (RMCE) was used to stably express HIV gag-pol. In principle, this involved ‘tagging’ a chromosomal location that was able to support good expression of a GFP cassette and then using cre-recombinase to exchange GFP for HIV gag-pol.
[0174]To tag a high expresser site, a mutant loxP site (with a mutation in the left inverted repeat), was cloned into the 3′ LTR of a gammaretroviral vector encoding a hygromycin-GFP fusion gene under the control of the CMV promoter (FIG. 3a). 293FT cells were infected at a low MOT and selected in hygromycin to obtain clones with a single copy of the gammaretroviral vector containing a target construct. One of these, clone 2G had a relatively high mean fluorescence ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| antibiotic resistance | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com