Production of fibrinogen in transgenic animals
a technology of fibrinogen and transgenic animals, which is applied in the field of blood coagulation, can solve the problems of limited acceptance and availability of such adhesives, autologous adhesives cannot be used in elective surgery, and the goal is elusiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example i
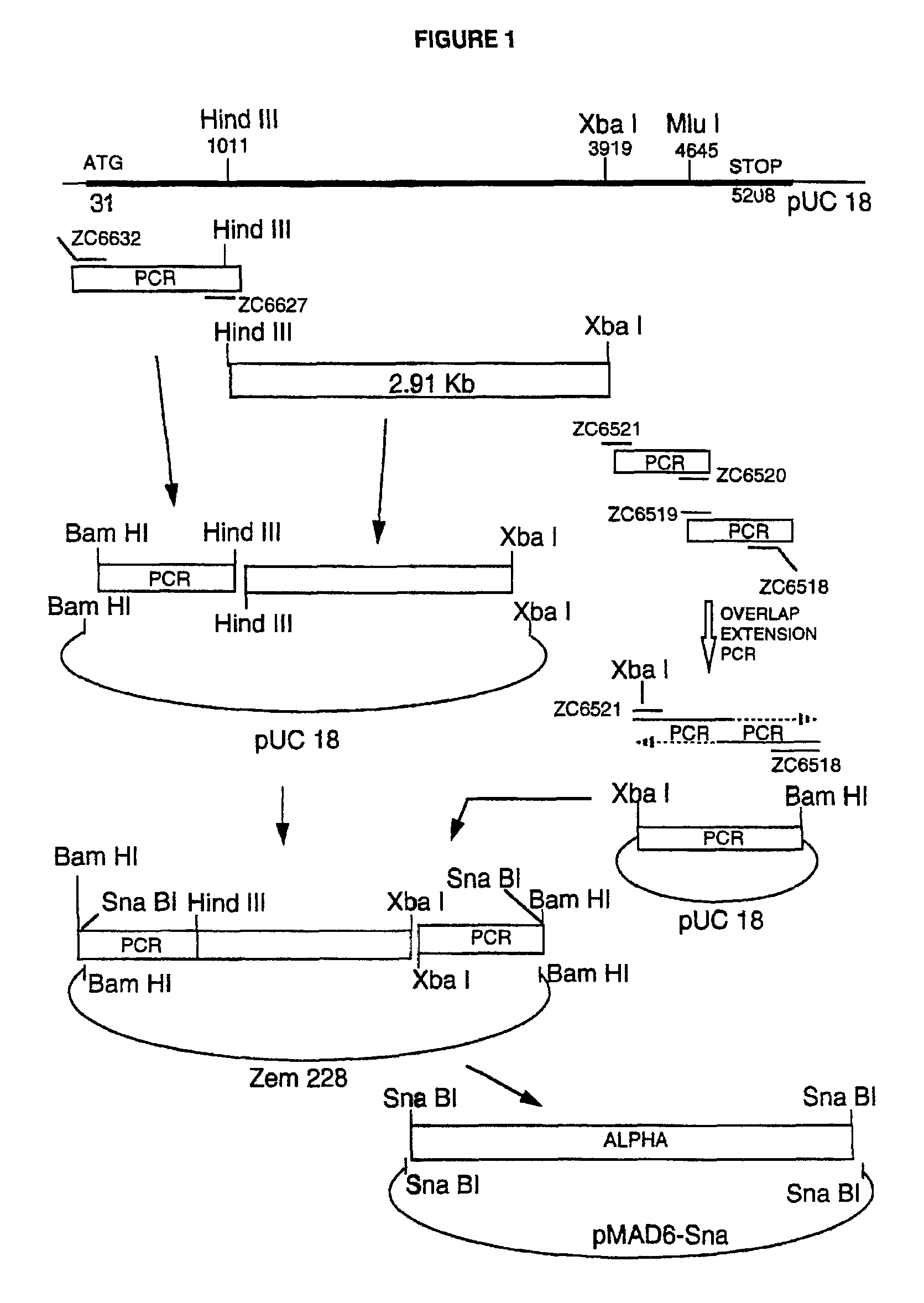
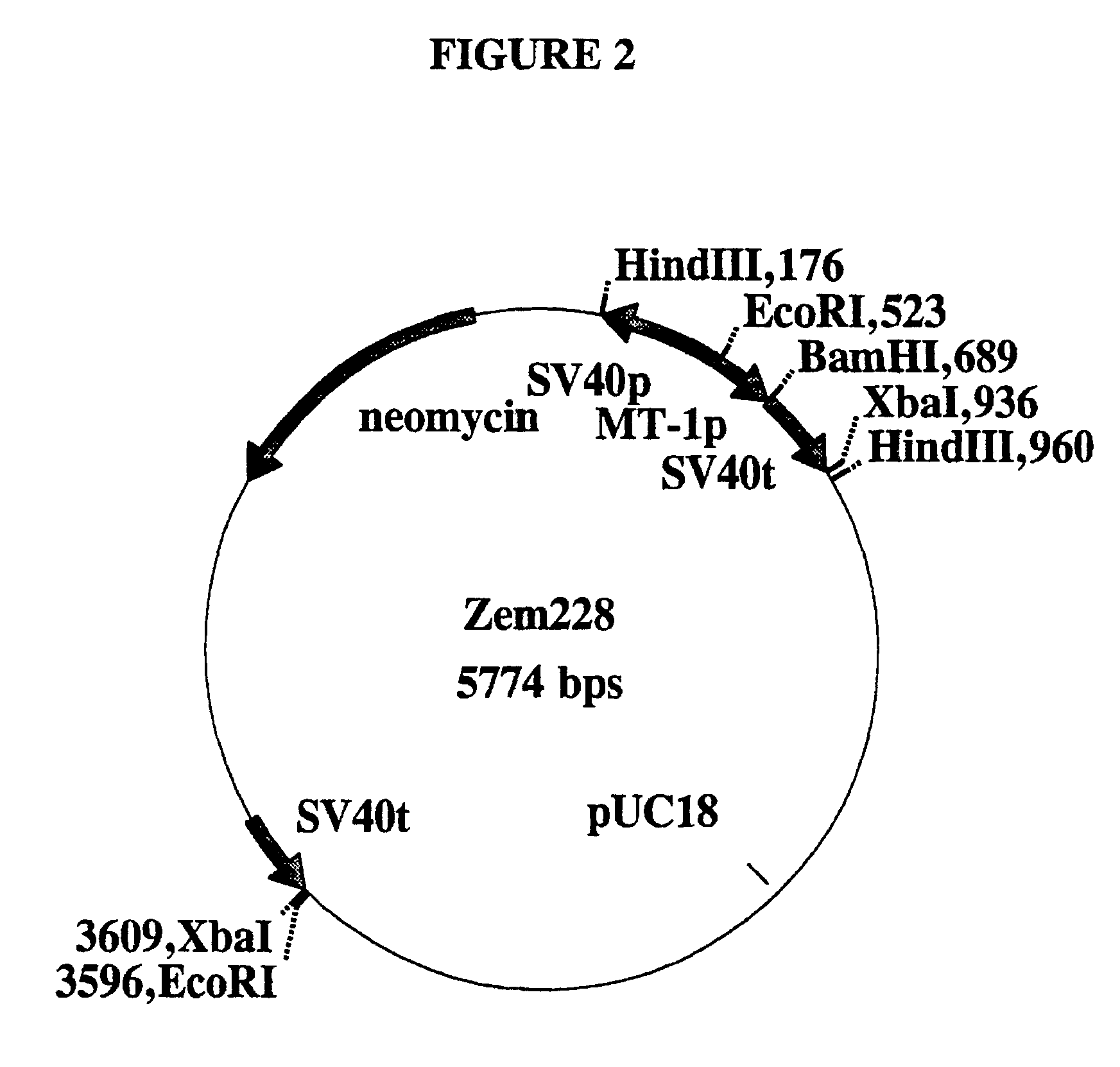
[0046]The multiple cloning site of the vector pUC18 (Yanisch-Perron et al., Gene 33:103-119, 1985) was removed and replaced with a synthetic double stranded oligonucleotide (the strands of which are shown in SEQ ID NO: 8 and SEQ ID NO: 27) containing the restriction sites Pvu I / Mlu I / Eco RV / Xba I / Pvu I / Mlu I, and flanked by 5′ overhangs compatible with the restriction sites Eco RI and Hind III. pUC18 was cleaved with both Eco RI and Hind III, the 5′ terminal phosphate groups were removed with calf intestinal phosphatase, and the oligonucleotide was ligated into the vector backbone. The DNA sequence across the junction was confirmed by sequencing, and the new plasmid was called pUCPM.
[0047]The β-lactoglobulin (BLG) gene sequences from pSS1tgXS (disclosed in WIPO publication WO 88 / 00239) were excised as a Sal I-Xba I fragment and recloned into the vector pUCPM that had been cut with Sal I and Xba I to construct vector pUCXS. pUCXS is thus a pUC18 derivative containing the entire BLG g...
example ii
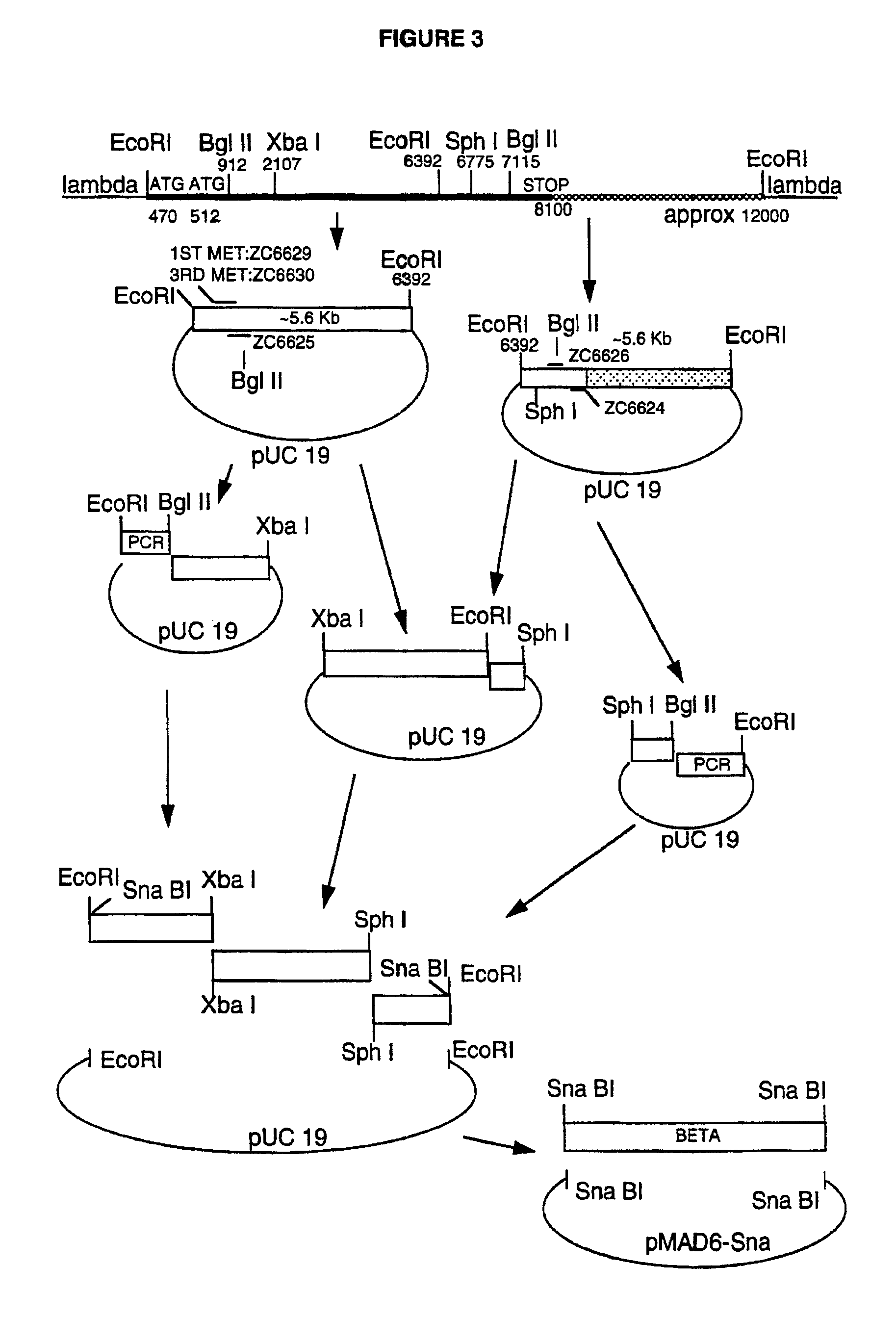
[0052]Clones encoding the individual fibrinogen chains were obtained from the laboratory of Dr. Earl W. Davie, University of Washington, Seattle. A genomic fibrinogen Aα-chain clone (Chung et al., 1990, ibid.) was obtained from the plasmid BS4. This plasmid contains the Aα clone inserted into the Sal I and Bam HI sites of the vector pUC18, but lacks the coding sequence for the first four amino acids of the Aα chain. A genomic Bβ-chain DNA (Chung et al., ibid.) was isolated from a lambda Charon 4A phage clone (designated βλ4) as two EcoRI fragments of ca. 5.6 Kbp each. The two fragments were cloned separately into pUC19 that had been digested with Eco RI and treated with calf intestinal phosphatase. The resulting clones were screened by digestion with the restriction enzyme Pvu II to distinguish plasmids with the 5′ and 3′ Bp inserts (designated Beta5′RI / puc and Beta3′RI / puc, respectively). Genomic γ-chain clones were isolated as described by Rixon et al. (Biochemistry 24: 2077-2086,...
example iii
[0059]Mice for initial breeding stocks (C57BL6J, CBACA) were obtained from Harlan Olac Ltd. (Bicester, UK). These were mated in pairs to produce F1 hybrid cross (B6CBAF1) for recipient female, superovulated females, stud males and vasectomized males. All animals were kept on a 14 hour light / 10 hour dark cycle and fed water and food (Special Diet Services RM3, Edinburgh, Scotland) ad libitum.
[0060]Transgenic mice were generated essentially as described in Hogan et al., Manipulating the Mouse Embryo: A Laboratory Manual, Cold Spring Harbor Laboratory, 1986, which is incorporated herein by reference in its entirety. Female B6CBAF1 animals were superovulated at 4-5 weeks of age by an i.p. injection of pregnant mares' serum gonadotrophin (FOLLIGON, Vet-Drug, Falkirk, Scotland) (5 iu) followed by an i.p. injection of human chorionic gonadotrophin (CHORULON, Vet-Drug, Falkirk, Scotland) (5 iu) 45 hours later. They were then mated with a stud male overnight. Such females were next examined ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap