Prepn process of microcapsule with included anticancer medicine
A technology for anticancer drugs and microcapsules, applied in the directions of microcapsules, capsule delivery, antitumor drugs, etc., can solve the problems of the permeability of microcapsules or microspheres, the difficulty of slow release performance, the inability to guarantee removal, and the environmental pollution, etc. Achieve excellent stability, good biocompatibility, and a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
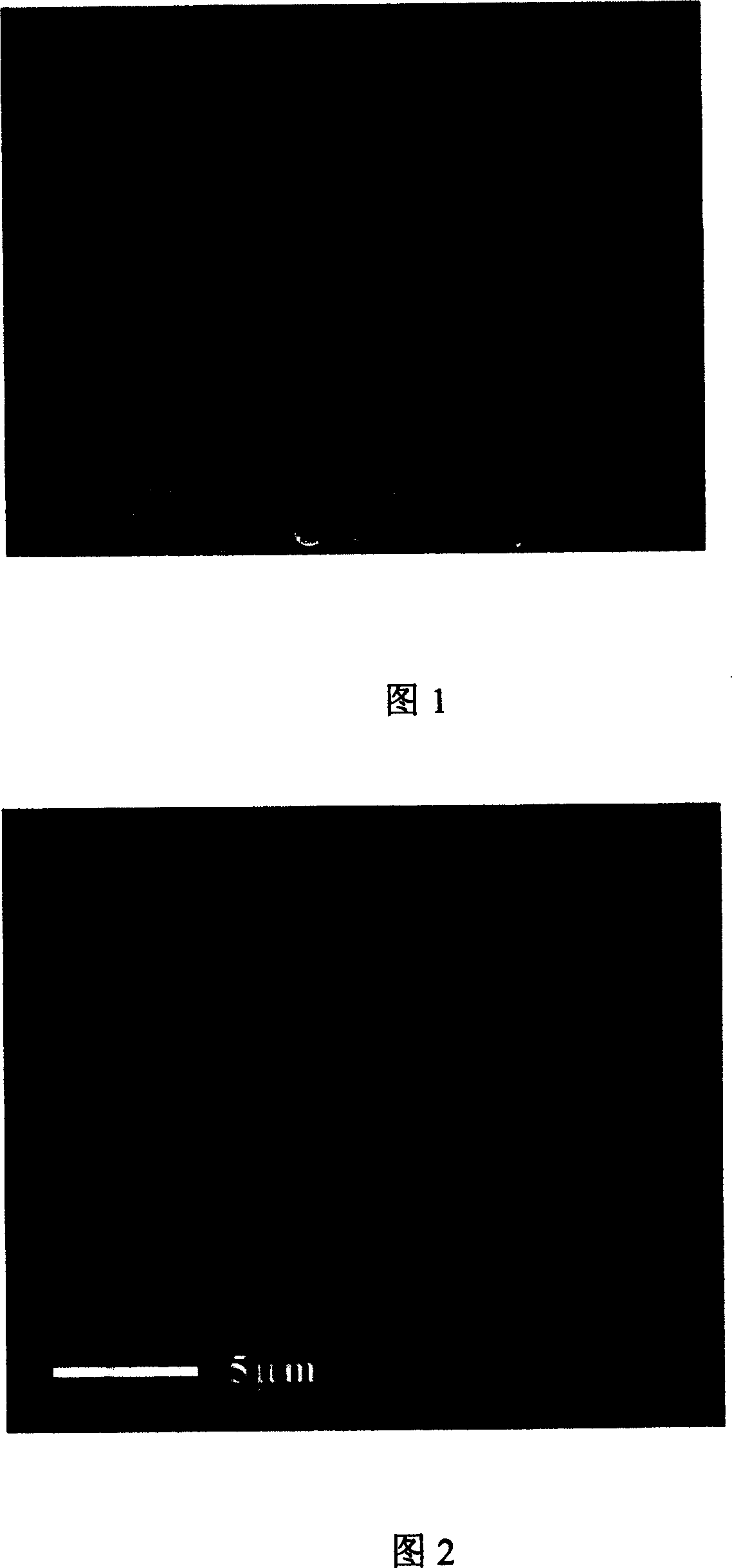
[0040] Mix 50mL of calcium nitrate solution with a concentration of 0.025M / L and 100mg of polystyrene sodium sulfonate (PSS), and after 10 minutes, quickly add 50mL of a sodium carbonate solution with a concentration of 0.025M / L. After 15 minutes, the generated calcium carbonate containing PSS (expressed as CaCO 3 (PSS)) The precipitate was collected by centrifugation, washed 3 times with water, and stored. Prepared CaCO 3 The scanning electron micrographs of (PSS) particles are shown in Fig. 1 .
[0041] 1 mL (solid content 5-10%) of the above-mentioned CaCO with a diameter of 2-10 μm 3 (PSS) microparticles were placed in a 2 mL centrifuge tube. (1) Centrifuge to remove supernatant, wash with water 3 times. (2) Add 1 mL of polyallylamine hydrochloride (PAH) solution to 0.2-0.5 M / L NaCl solution, and shake the centrifuge tube. After 10 min, wash with water 3 times to remove excess PAH, thus the CaCO 3 (PSS) surface adsorbed a layer of PAH (expressed as CaCO 3 (PSS)-PAH)...
example 2
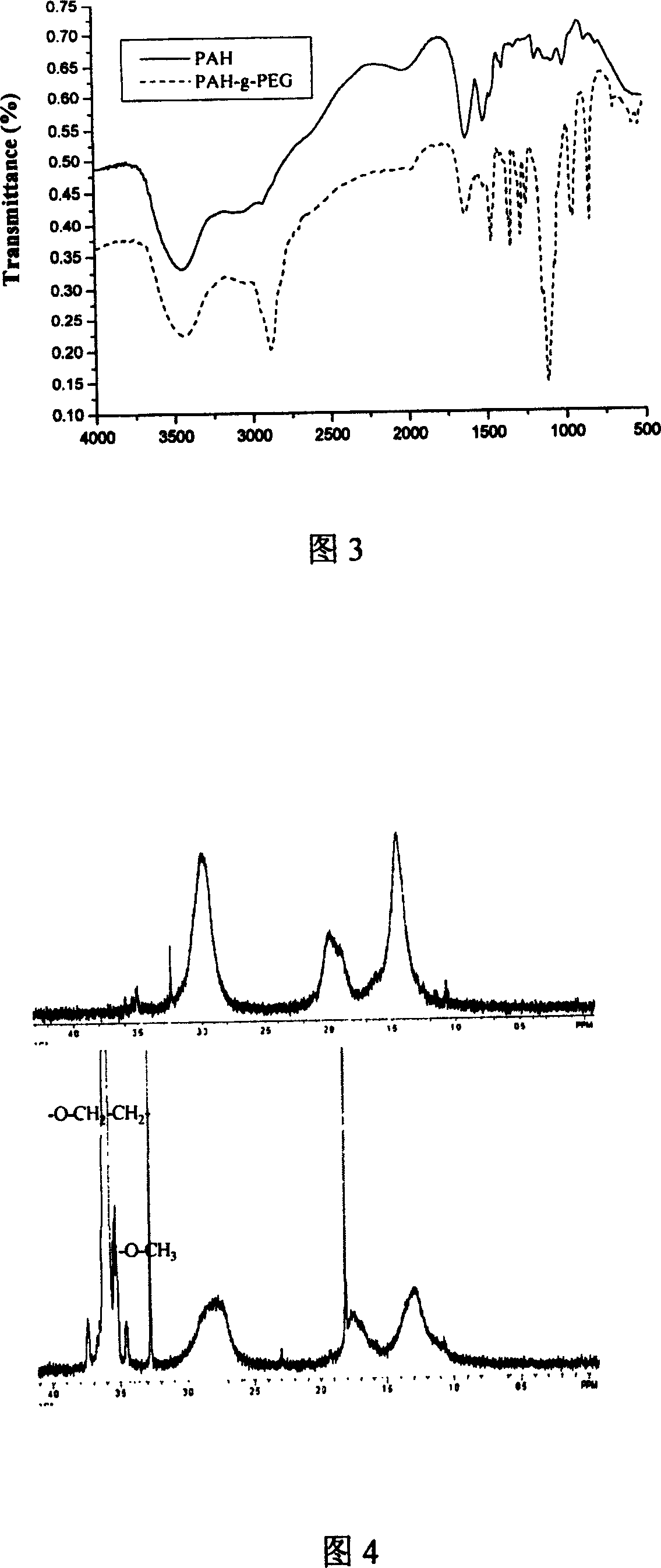
[0044] (1) Dissolve 2 g (10 mmol) of p-toluenesulfonyl chloride (TsCl) in 50 mL of pyridine. Dissolve 5 g (2.5 mmol) of monomethoxypolyethylene glycol in 50 mL of dichloromethane, and add the above-mentioned TsCl solution in pyridine with stirring. React for 12 hours. The reaction product was extracted by adding 50mL of 3mol / L hydrochloric acid, and the organic layer was added with 2.5g NaHCO 3 , stirred and filtered to obtain crude polyethylene glycol p-toluenesulfonate (PEG-Ts). The crude PEG-Ts was dissolved in 5 mL THF with stirring, and precipitated with ether three times. The pure PEG-Ts was obtained after vacuum drying. (2) Take 2.38g (1.09mmol) of PEG-Ts, add 0.4335g (0.0062mmol) of PAH in borax solution (pH=9.18), stir, and react at room temperature for 24h. (3) Dialyzing the resulting product for a sufficient time to remove small molecule impurities. After lyophilization, the final product polyallylamine grafted with polyethylene glycol (PAH-g-PEG) was obtained....
example 3
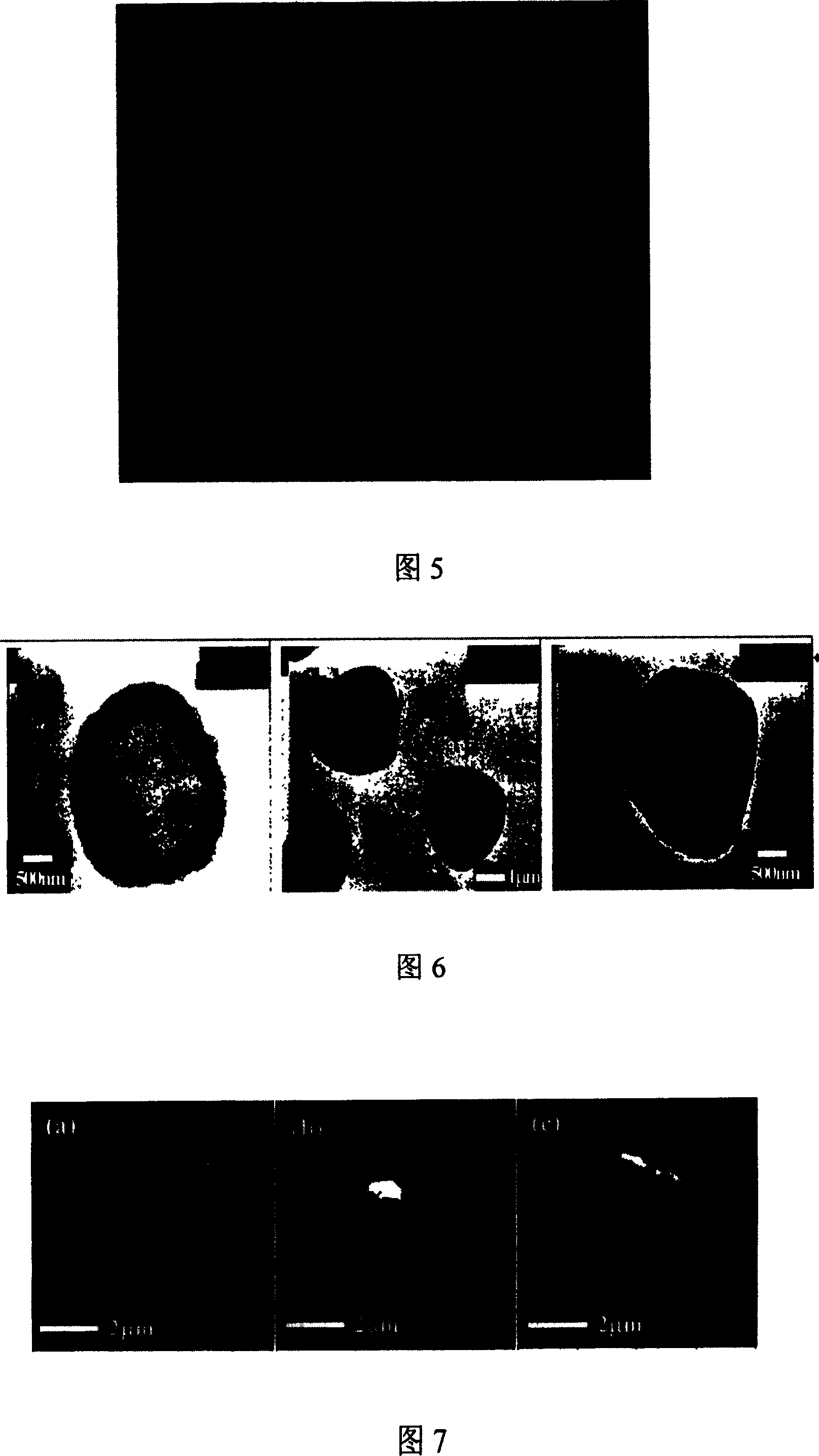
[0047] Microcapsules were prepared as in Example 1, but the last layer replaced PAH with rhodamine-labeled PAH-g-PEG to obtain CaCO 3 (PSS) / (PAH / PSS) 4 / PAH-g-PEG microcapsules. After 10 seconds, observed under a confocal laser microscope, it was found that the rhodamine-labeled PAH-g-PEG was assembled onto the microcapsules, which had a hollow and complete structure (Fig. 5).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com