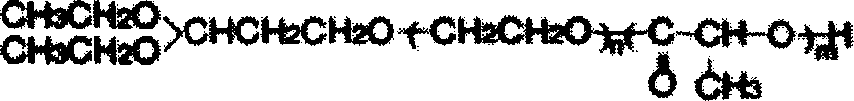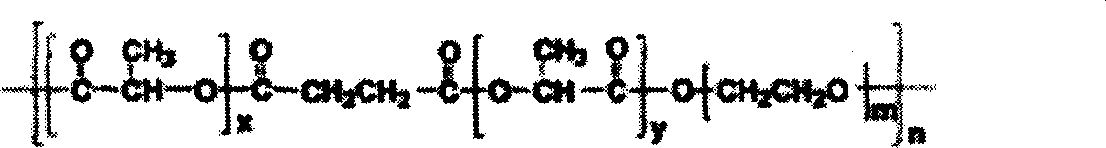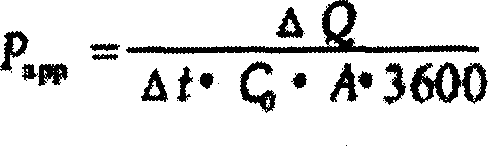Block polymer for eyes
A technology of block polymers and di-block polymers, applied in drug combinations, medical preparations of non-active ingredients, sensory diseases, etc., can solve the problems of low corneal permeability and low bioavailability, and reduce side effects , improve curative effect, improve the effect of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 This embodiment is the preparation of ophthalmic pirenzepine polymer micelle gel
[0045] Materials and Methods
[0046] D, L-lactide: purchased from Sigma, recrystallized three times with anhydrous ethyl acetate at 60°C before use, and at room temperature in P 2 o 5 Dry under medium vacuum for 24 hours. Both stannous octoate and methyl polyethylene glycol (molecular weight 2000) were purchased from Sigma Company.
[0047] 1. Synthesis of methyl polyethylene glycol-polylactic acid (mPEG-PLA) diblock polymer:
[0048] The ring-opening polymerization method was used to prepare methyl polyethylene glycol-polylactic acid block polymers with a mass ratio of 75 / 25. That is: Weigh 7.5g of methyl polyethylene glycol and 2.5g of lactide, place them in a closed reactor, raise the temperature to 120-140°C under nitrogen flow to melt the solid, add 50mg of stannous octoate, and raise the temperature Reaction at 150-180°C for 3-6 hours. After cooling, a white soli...
Embodiment 2
[0057] Embodiment 2 This embodiment is the isolated corneal penetration experiment of ophthalmic pirenzepine polymer micelle gel
[0058] Rabbits were taken and anesthetized to death. The eyeballs were enucleated and placed in the center. Make a transverse cut along the sclera 2 mm from the corneal edge to separate the cornea. The lens, vitreous body and other tissues at the back of the eyeball were removed, and the iris was peeled off to obtain a cornea with a 2mm scleral ring. Corneal permeation experiments were started within 20 min after the animals were sacrificed. Adopt osmotic diffusion device, including supply pool and accept pool, maintain the shape of the isolated cornea and fix it between the two pools, so that the epithelial layer faces the supply pool. Add pirenzepine hydrochloride to be dissolved in the gel 2mL that diluent obtains in embodiment 1 in the supply pool or ophthalmic pirenzepine polymer micelle gel, accept the pool to be Ringer's solution, and who...
Embodiment 3
[0063] Example 3 Pharmacokinetics of pirenzepine polymer micellar gel, eye drops in rabbit aqueous humor
[0064] The 40 rabbits were randomly divided into 8 groups according to the sampling time, with 5 rabbits in each group. The prescription was changed every two weeks for a total of 2 times (pirenzepine hydrochloride ophthalmic gel and pirenzepine polymer micelles ophthalmic gel, respectively). During the administration, 2 drops (100 μL) of the prepared pirenzepine eye drops were dripped in the conjunctival sac of one eye respectively, and no medicine was used in the left eye. At 0.5, 1, 2, 4, 8, 12, 24, and 48 hours after eye drops, 0.2ml of aqueous humor was drawn from the right eye at 8 time points in total. Take 0.1 mL of aqueous humor, add 0.1 mL of methanol, and vortex to mix to precipitate proteins and impurities. Centrifuge at a speed of 16000r / min for 10min, take the supernatant, inject it into a high-performance liquid chromatograph, record the peak area, and ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com