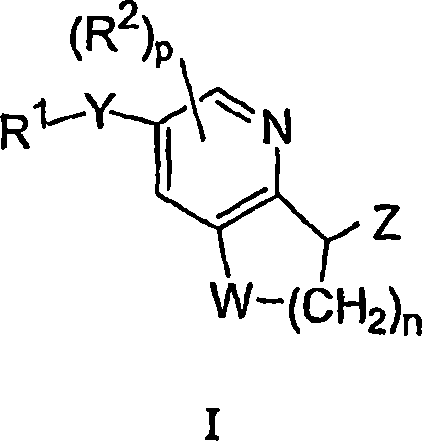
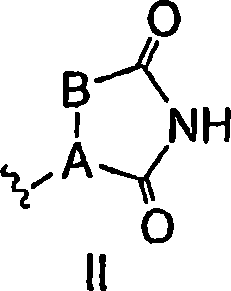
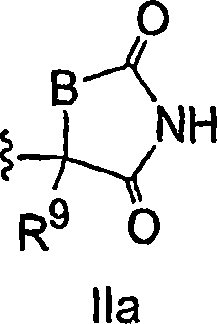
Antidiabetic bicyclic compounds
A compound and heterocyclic technology, applied in the field of bicyclic compounds, can solve the problems of lipid distribution with side effects and failure to significantly improve lipid metabolism, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0220]
[0221] To Intermediate 5-3 (0.1 mmol, 36 mg), CuI (0.02 mmol, 4 mg), N,N-dimethylglycine HCl salt (0.06 mmol, 8.4 mg), 2-methyl-4-fluoro-phenol ( 0.15mmol, 19mg) and Cs 2 CO 3 (0.47 mmol, 153 mg) was added dioxane (1 mL). The reaction was heated at 95°C overnight, then filtered. The filtrate was neutralized with TFA (0.5 mL), then concentrated. The residue was subjected to reverse phase HPLC (YMC-Pack Pro C185 micron, 20%-80% CH 3 CN / H 2 O / 0.1% TFA) to give pure product as a solid. LC-MS determination of C 18 h 16 FN 2 o 3 S[M+H + ]: theoretical value 359.1, measured value 359.0.
Embodiment 2
[0223]
[0224] To intermediate 5-3 (0.1mmol, 36mg), CuI (0.02mmol, 4mg), N,N-dimethylglycine HCl salt (0.06mmol, 8.4mg), 2,4-dichloro-phenol (0.15mmol , 25mg) and Cs 2 CO 3 (0.47 mmol, 153 mg) was added dioxane (1 mL). The reaction was heated at 95°C overnight, then filtered. The filtrate was acidified with TFA (0.5 mL), then concentrated. The residue was subjected to reverse phase HPLC (YMC-Pack Pro C185 micron, 20%-80% CH 3 CN / H 2 O / 0.1% TFA) to give pure product as a solid. LC-MS determination of C 17 h 13 Cl 2 N 2 o 3 S[M+H + ]: theoretical value 395.0, measured value 394.9.
Embodiment 3
[0226]
[0227] To a solution of Intermediate 5-5 (25 mg, 0.1 mmol) in DMF (0.5 mL) was added Cs sequentially 2 CO 3 (0.3mmol, 98mg), 3-chloro-2-fluoro-5-(trifluoromethyl)pyridine (0.15mmol). The reaction was heated at 50 °C for 1 h with CH 3 CN (1 mL) was diluted, then acidified with trifluoroacetic acid (0.4 mL). The mixture was subjected to reverse phase HPLC (YMC-Pack Pro C185 microns, 20%-80% CH 3 CN / H 2 O / 0.1% TFA) to give pure product as a solid. LC-MS determination of C 17 h 12 CIF 3 N 3 o 3 S[M+H + ]: theoretical value 430.0, measured value 430.0.
[0228] Intermediate 6-1
[0229]
[0230] This intermediate was prepared from cyclohexanone ethyl acetate according to the method of Intermediate 5-1. LC-MS determination of C 13 h 17 INO 2 [M+H + ]: theoretical value 346.0, measured value 346.0.
[0231] Intermediate 6-2
[0232]
[0233] This intermediate was prepared from Intermediate 6-1 by converting Intermediate 5-1 to 5-2.
[0234] P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com