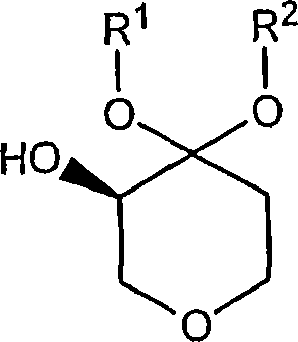
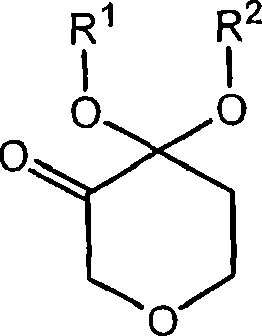
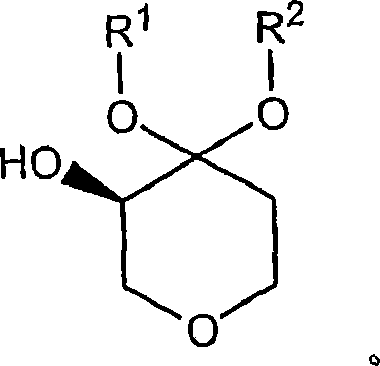
Process for the preparation of (r)-4,4-dialkoxy-pyran-3-ols such as (r)-4,4-dimethoxy-pyran-3-ol
A dimethoxy, dialkoxy technology, used in the preparation of (R)-4,4-dialkoxy-pyran-3-ols such as (R)-4,4-dimethoxy- In the field of pyran-3-ol, it can solve the problems of low yield, expensive use, and low enantiomeric purity of products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (R)-4,4-Dimethoxy-pyran-3-ol : Prepare the following materials: 3.18 kg (2.9 liters) of dimethoxypyrone in water containing 0.62 kg of dimethoxypyrone (3.88 mol), 0.62 g of KRED101 (5.9 MU) dissolved in 62 ml of 0.5 mol / A solution of 1 liter of phosphate buffer (pH6.5), 1.86 g of GDH (37.2 MU) dissolved in 62 ml of 0.5 mol / L of phosphate buffer (pH6.5), 0.74 g of NADP+disodium salt (0.94 mmol ) in 62 ml of 0.5 mol / L phosphate buffer (pH 6.5) and 0.8 kg of glucose (4.48 mol) in 2 liters of 1.5 mol / L phosphate buffer (pH 6.5). This glucose solution was placed in a container and 3.18 kg of an aqueous solution of dimethoxypyrone was added to obtain a final buffer concentration of 0.5 mol / liter. The reaction was maintained at 35°C. Add the NADP+ solution and the two enzyme solutions. The final reaction volume was 7.5 kg (6.2 liters). The reaction was monitored by the drop in pH and the pH was gradually adjusted from 6.0 to 6.5 by adding about 0.5 L of 2.5 mol / L KHCO3 so...
Embodiment 2
[0050] (R)-4,4-Dimethoxy-pyran-3-ol : Prepare the following materials: an aqueous solution of 204 kg (193 liters) of dimethoxypyrone containing 38.3 kg of dimethoxypyrone (239 mol), 38.4 grams of KRED101 (365MU) dissolved in 3.85 liters of 0.5 mol / L phosphoric acid A solution of salt buffer (pH 6.5), a solution of 115.5 g of GDH (2310 MU) dissolved in 3.85 liters of 0.5 mol / L phosphate buffer (pH 6.5), a solution of 47.6 g of NADP + disodium salt (60 mmol) in A solution of 3.85 liters of 0.5 mol / L phosphate buffer (pH 6.5) and 49.8 kg of glucose (277 mol) dissolved in 32 liters of 1.5 mol / L phosphate buffer (pH 6.5). This glucose solution was placed in a container and 204 kg of an aqueous solution of dimethoxypyrone was added to obtain a final buffer concentration of 0.5 mol / liter. The reaction was maintained at 35°C. Add the NADP+ solution and the two enzyme solutions. The reaction was monitored by pH drop and by adding approximately 83 L of 2.5 mol / L KHCO during the react...
Embodiment 3
[0052] Extraction of (R)-4,4-dimethoxy-pyran-3-ol : 1.46 kg KCl (~2 mol / L) was added to the reaction mixture of Example 1 (about 9.7 L containing 620 g (R)-4,4-dimethoxy-pyran-3-ol). Then, 1.5 batch volume (BV) of acetonitrile was added to extract the (R)-4,4-dimethoxy-pyran-3-ol product, followed by 0.5 BV of toluene to dry the organic layer. Separate the organic and aqueous layers into separate drums. The aqueous layer was then back extracted with 1.5 BV acetonitrile and 0.5 BV toluene. (The solvent used for all extractions was FisherPak solvent). The charge and volume distributions for the two extractions are summarized in the table below:
[0053] Charge Used - First Extraction
[0054] Capacity (L)
Standing time (minutes)
14.6
n / a
4.9
~20
[0055] Volume Distribution - First Extraction
[0056] Volume (L)
19.6
8.7
[0057] Char...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com