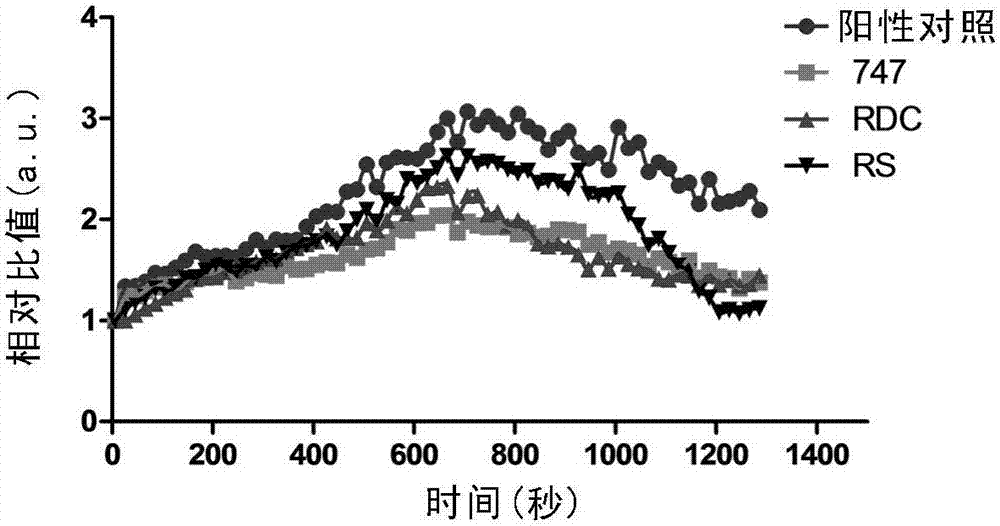
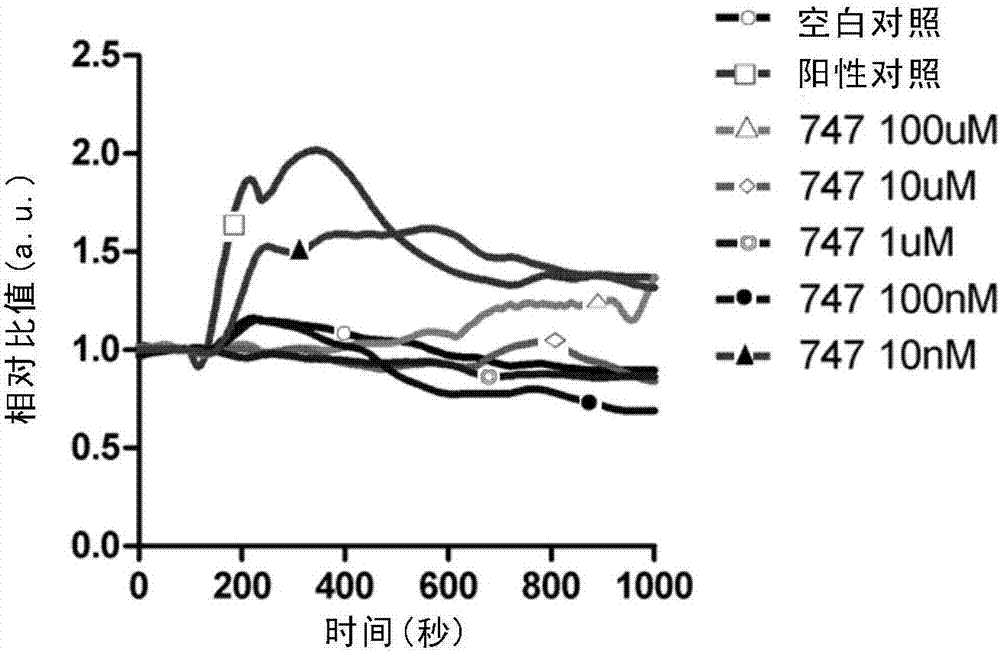
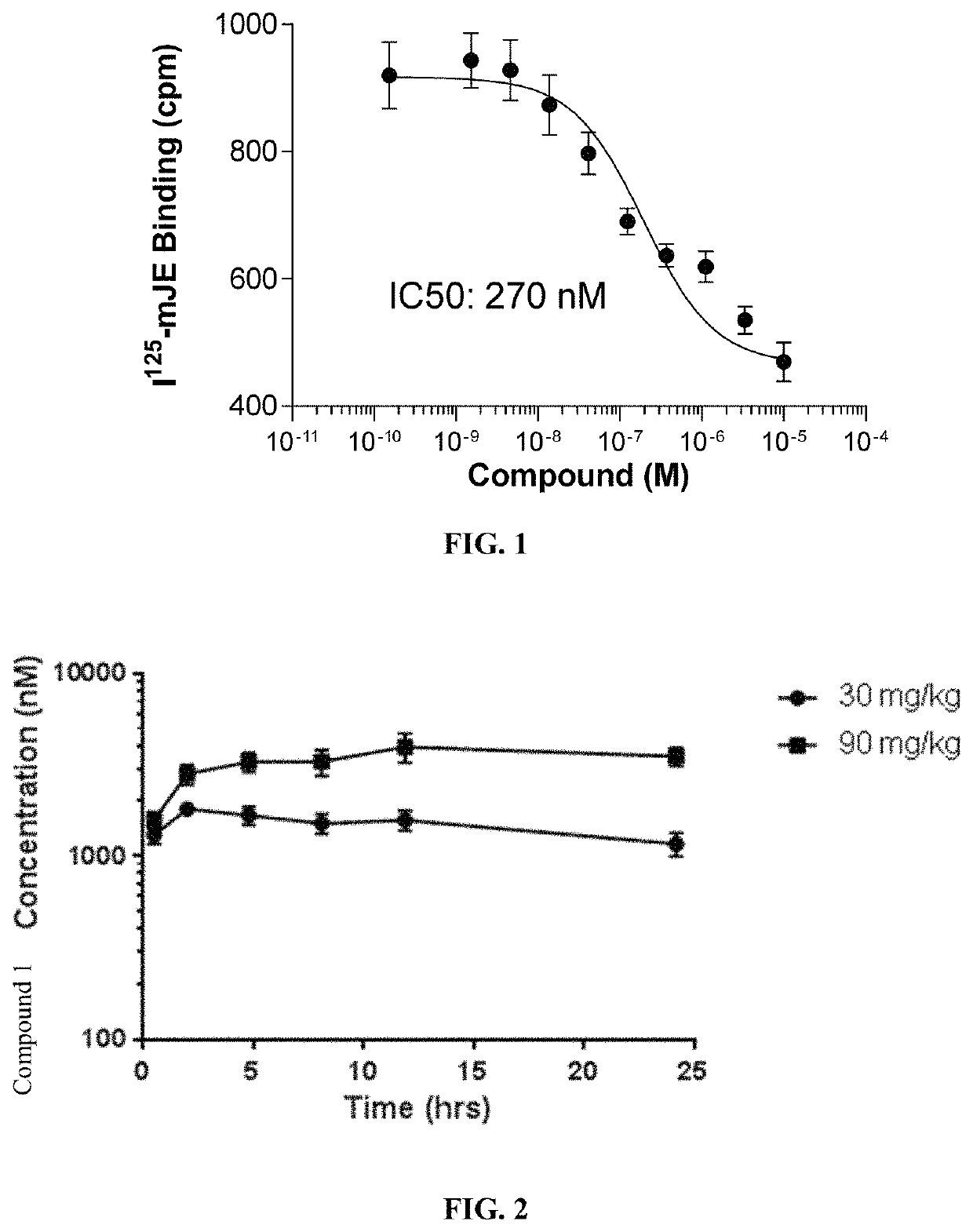
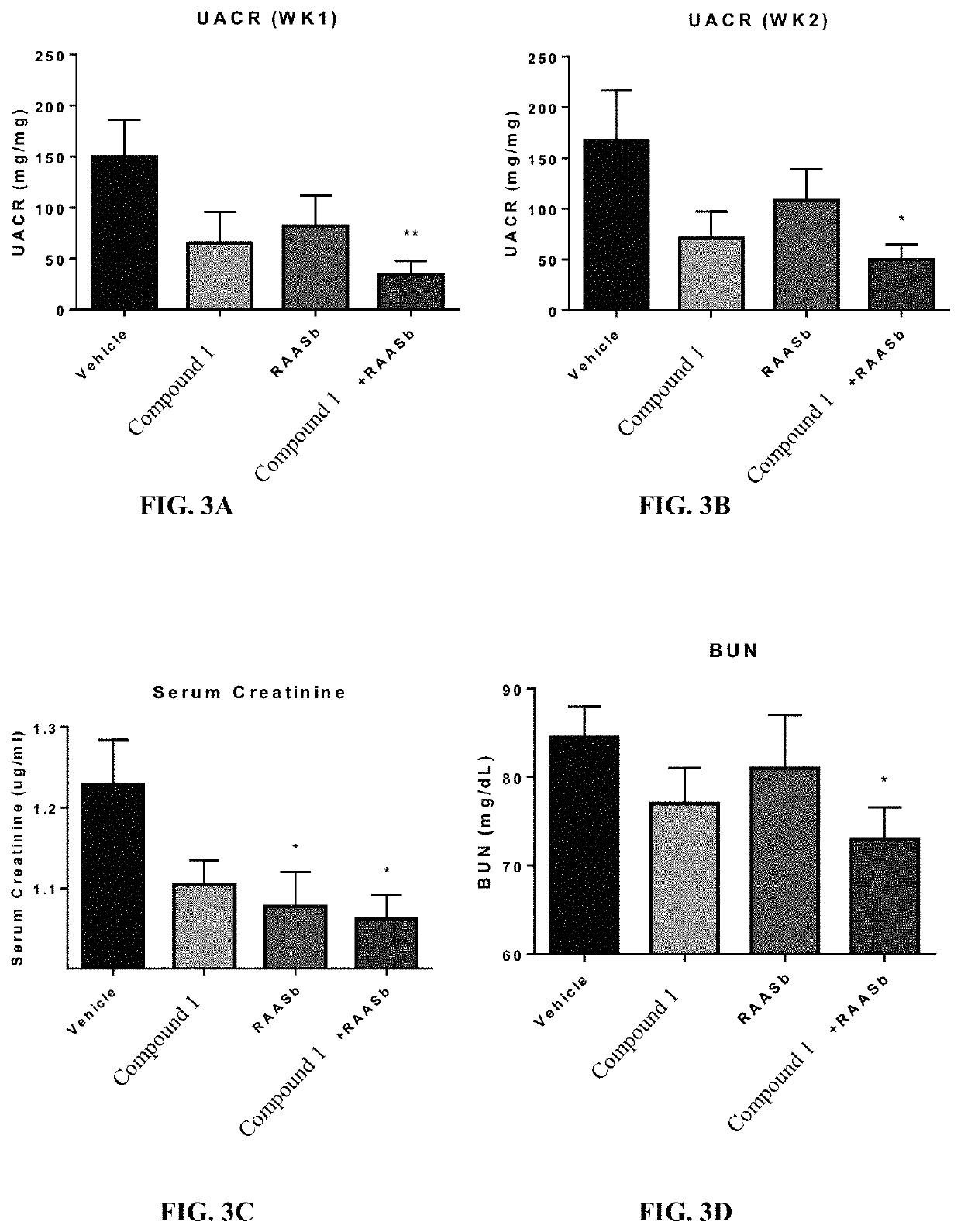
Patents
Literature
32 results about "Ccr2 antagonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CCR2 antagonists. Inhibition of CCR2 has been considered as a target for multiple therapeutic diseases including autoimmune disease, atherosclerosis, pain, and metabolic disease, based in part on the critical role this receptor plays on monocyte migration.
Heteroaryl sulfonamides and CCR2
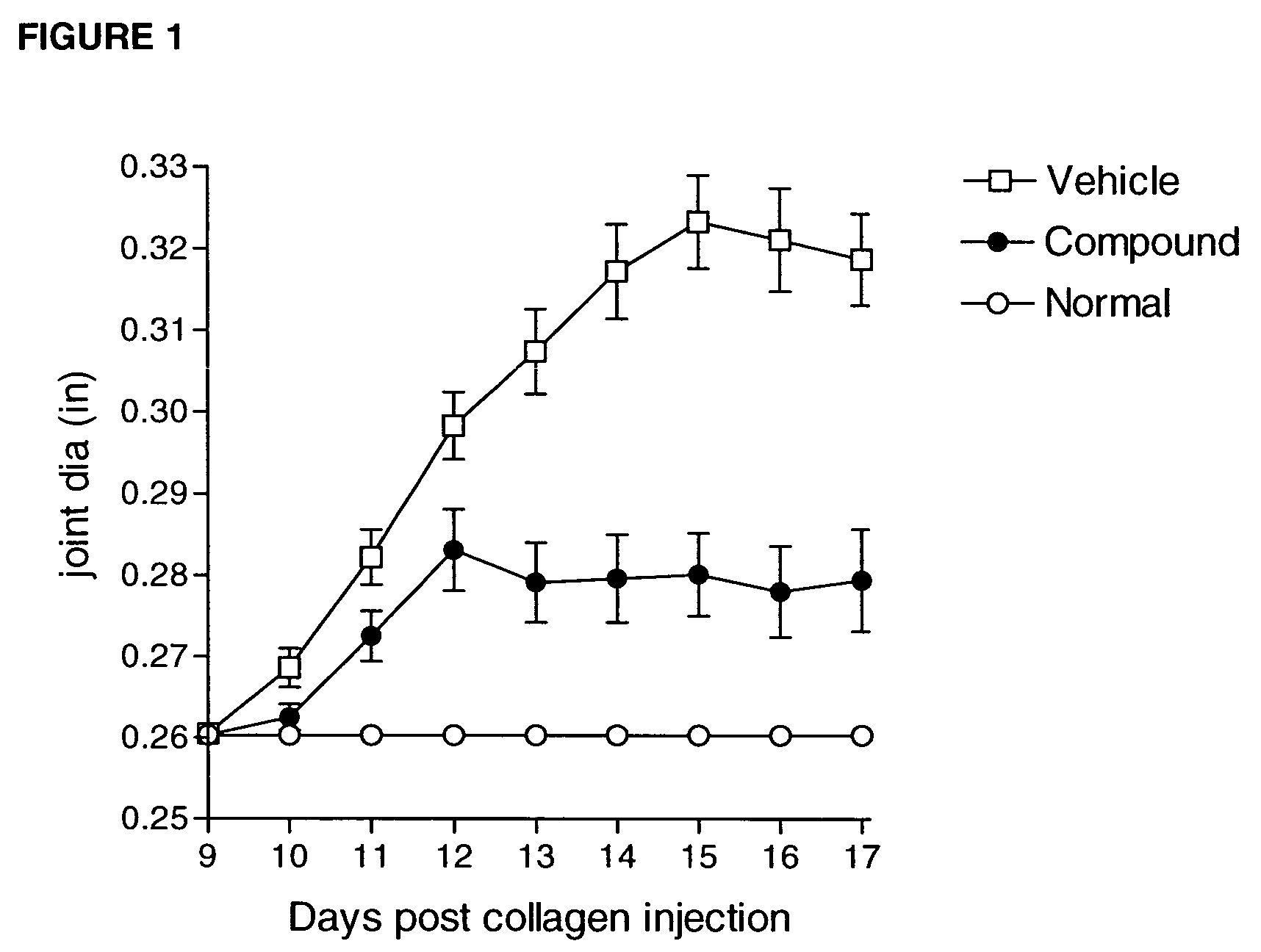
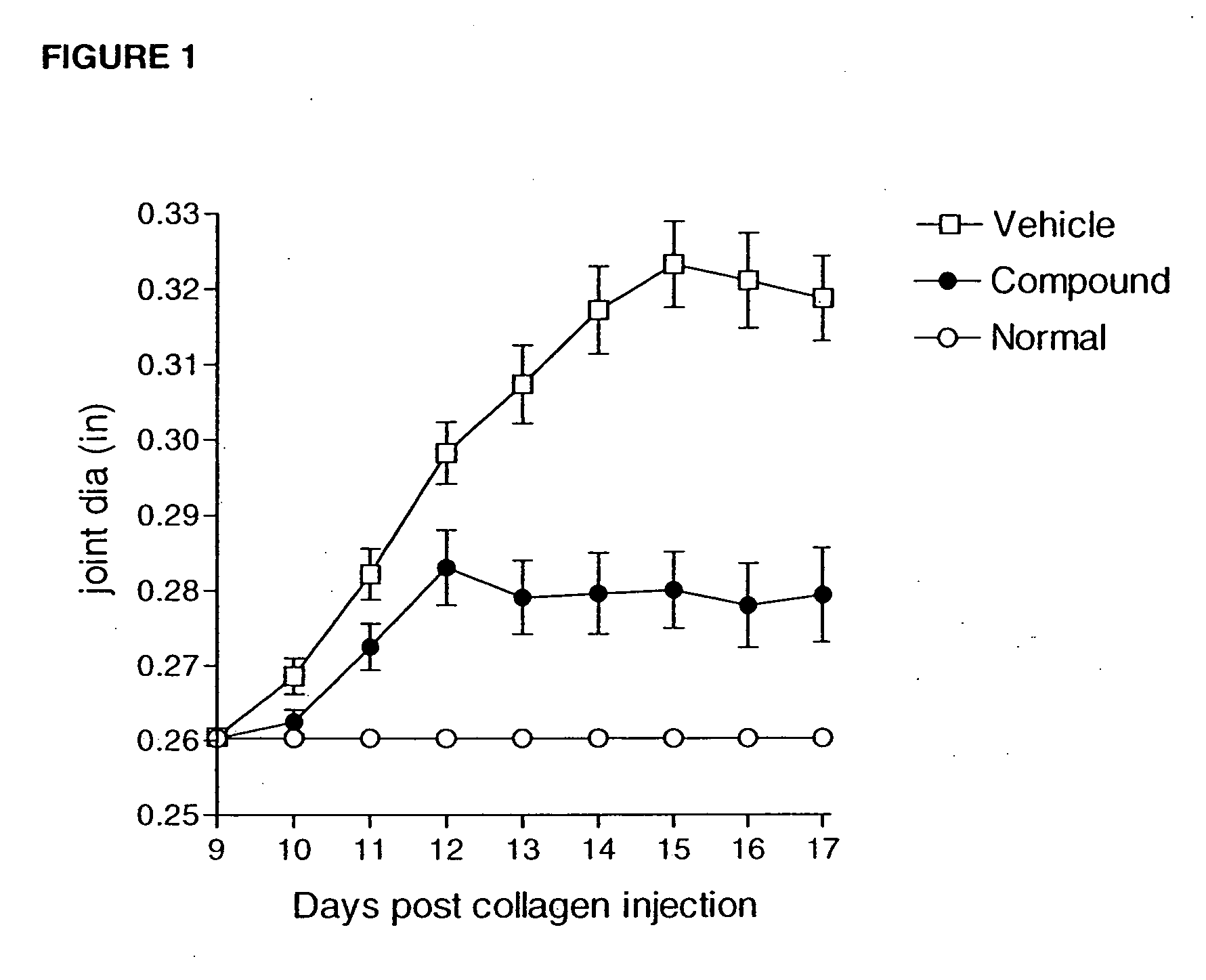
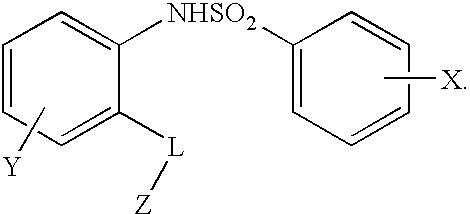
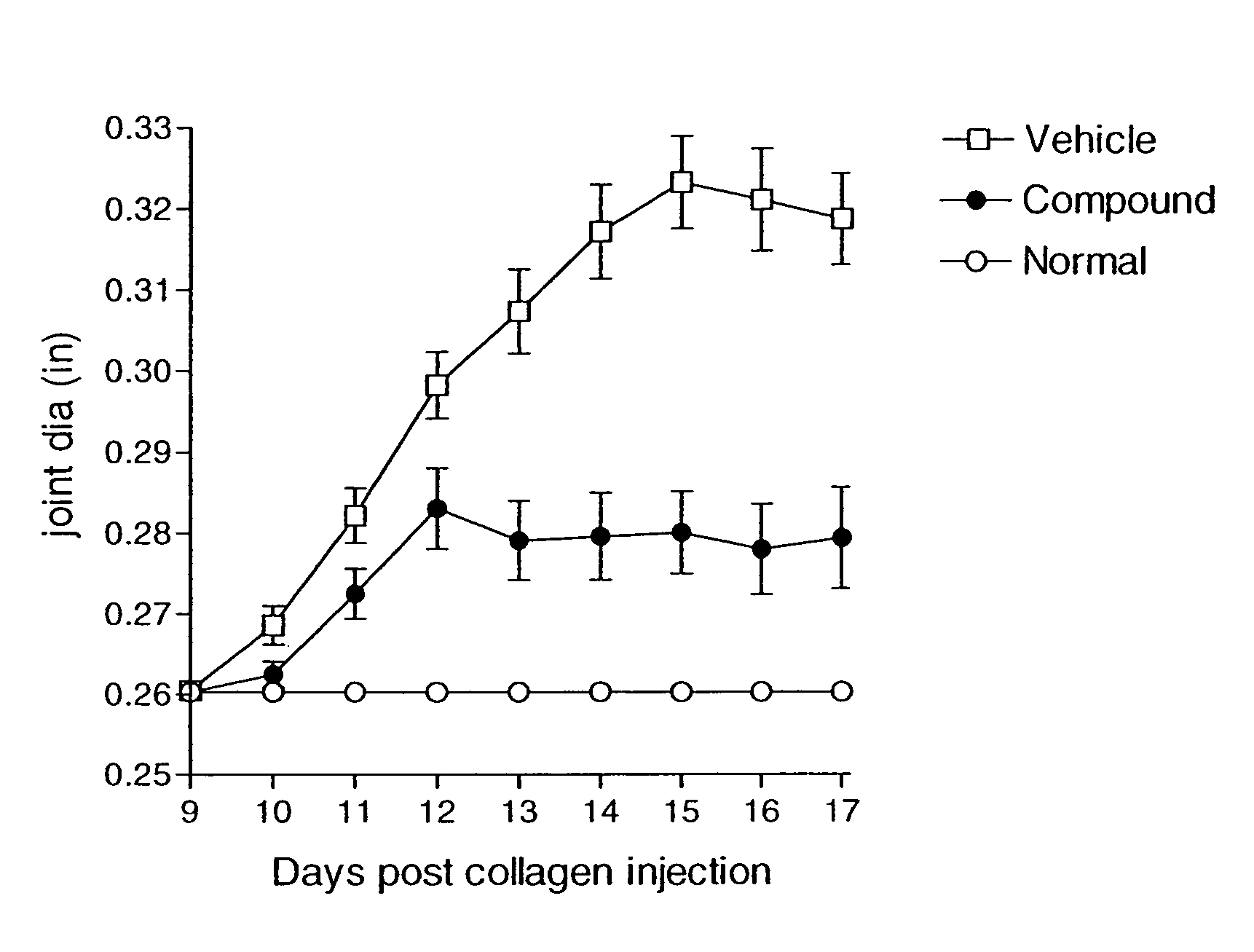
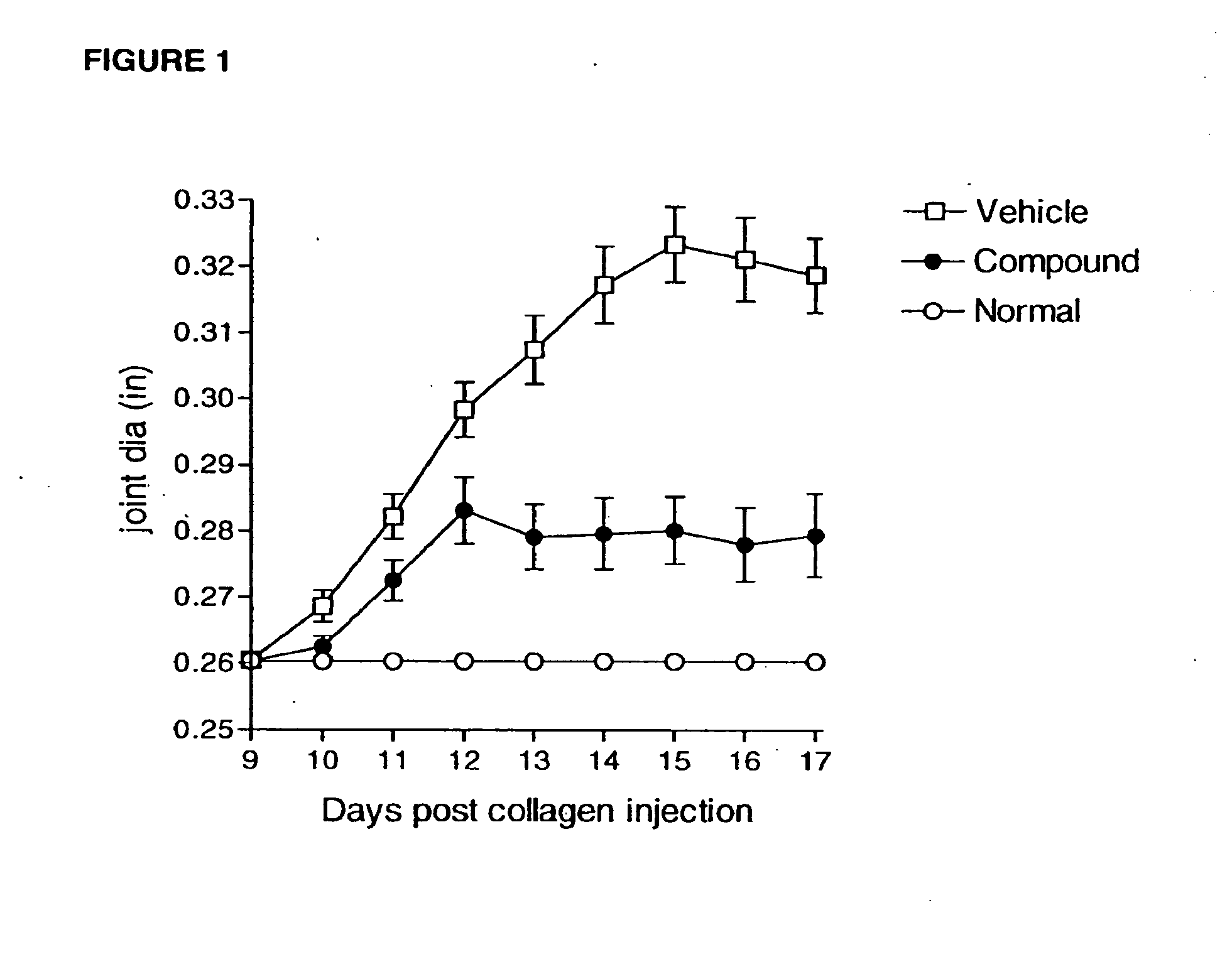
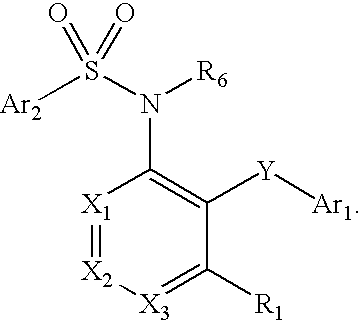
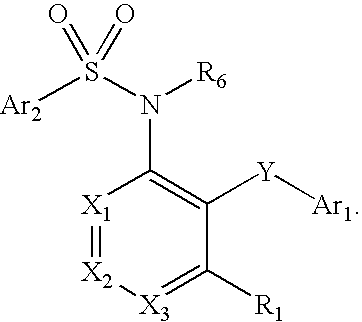
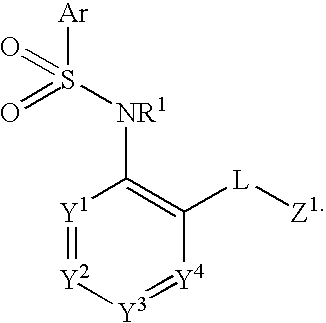
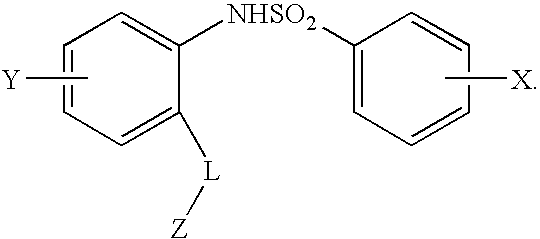
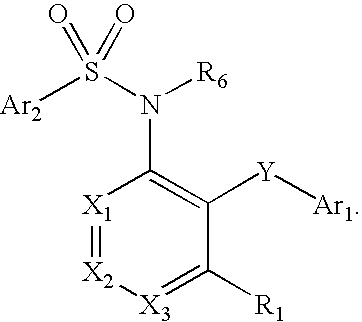
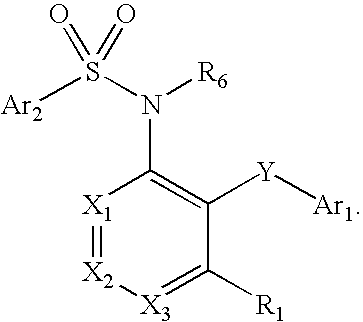
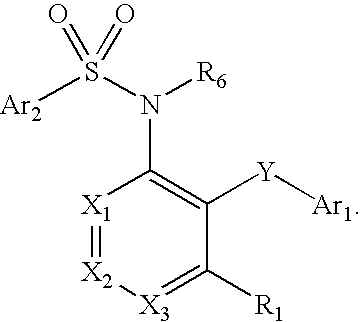
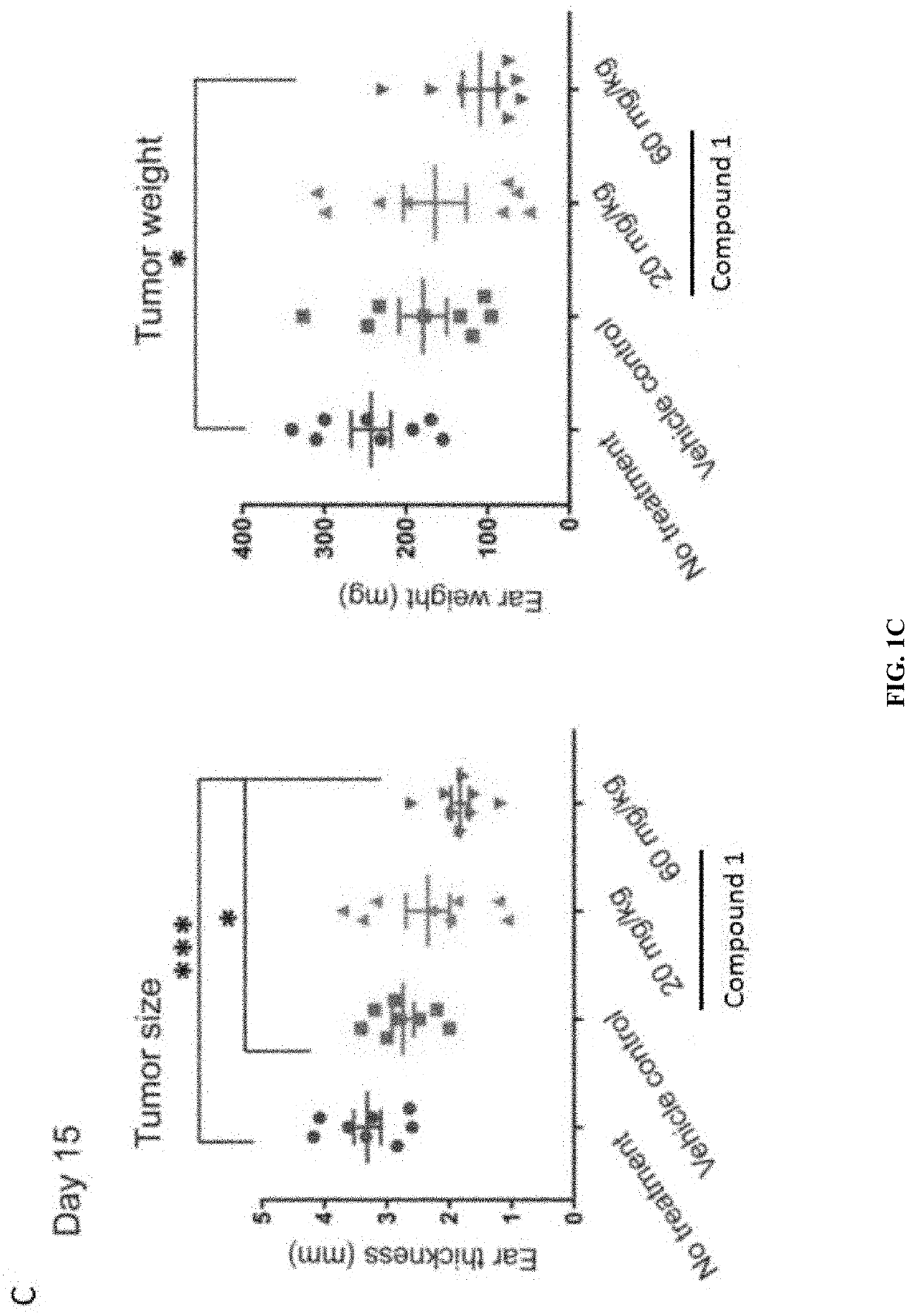
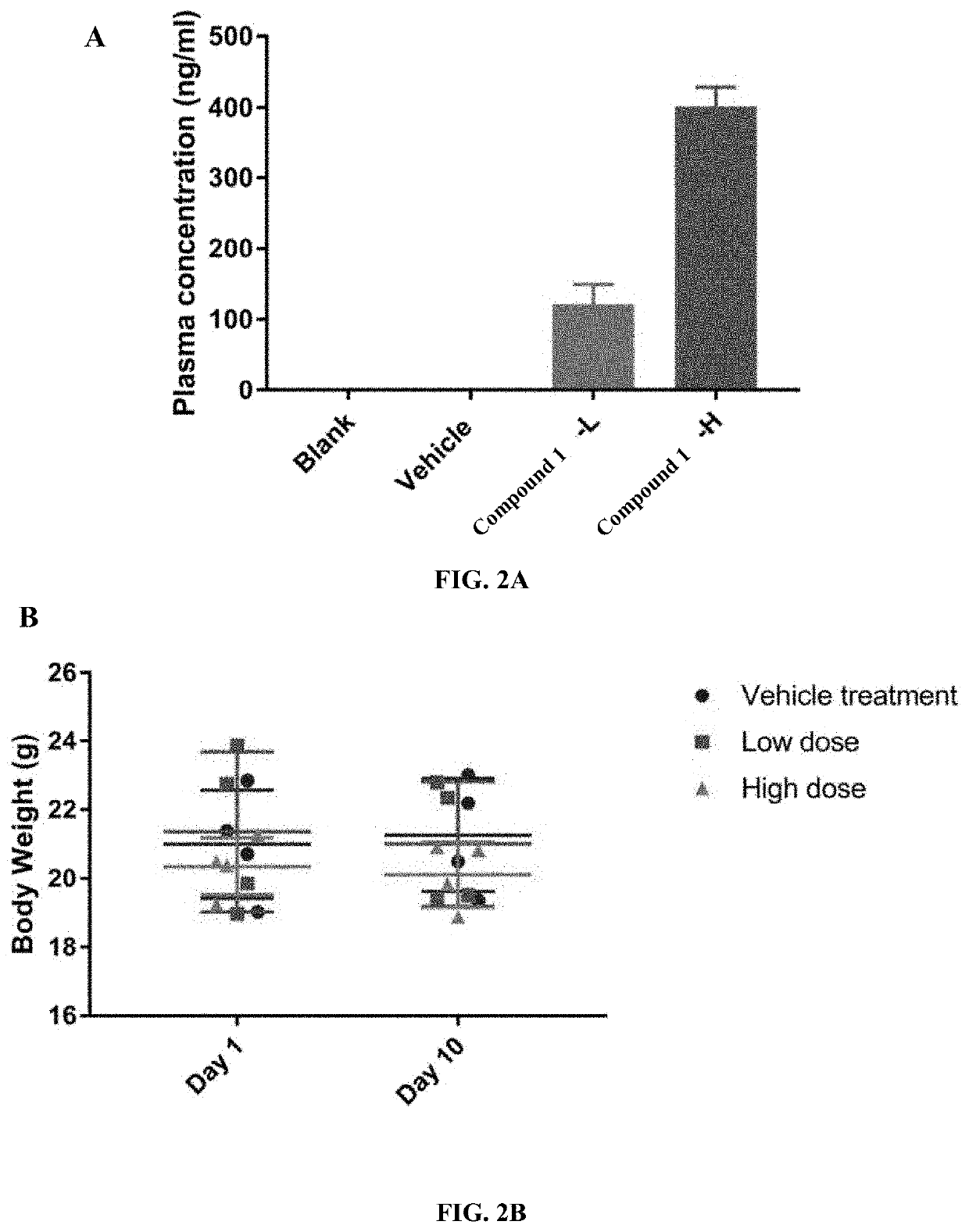
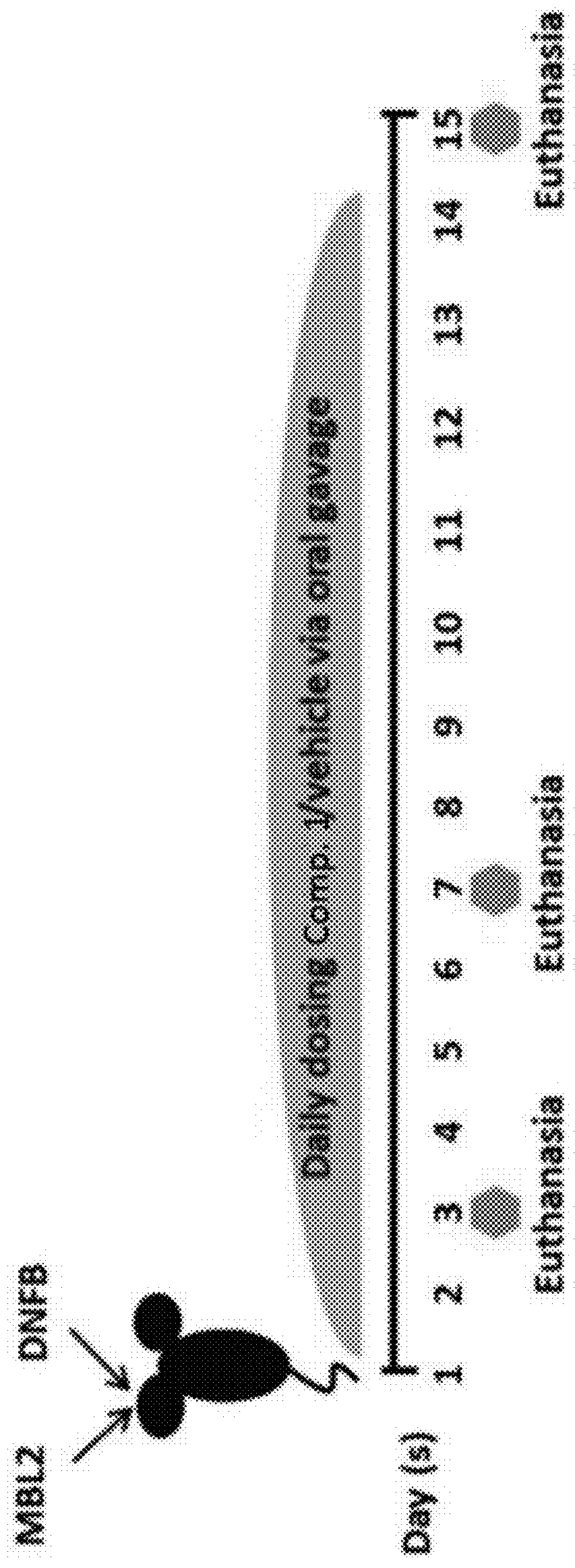
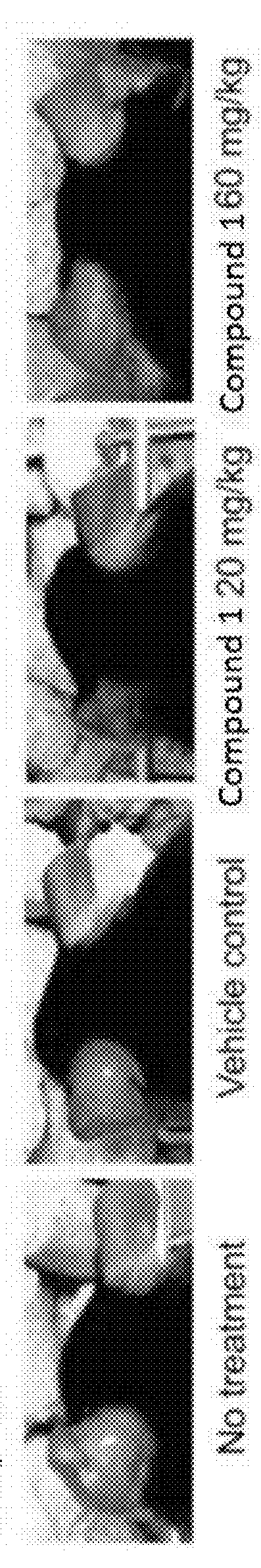
Compounds are provided that act as potent antagonists of the CCR2 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
Heteroaryl sulfonamides and CCR2/CCR9
Compounds are provided that act as potent antagonists of the CCR2 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
CCR2 inhibitors and methods of use thereof
Compounds are provided that act as potent antagonists of the CCR2 receptor. These compounds are useful for treating inflammation, a hallmark disease for CCR2. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
Heteroaryl sulfonamides and CCR2
Compounds are provided that act as potent antagonists of the CCR2 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
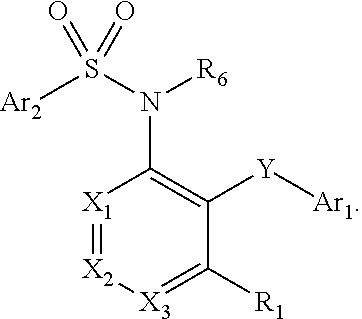
Fused heteroaryl pyridyl and phenyl benzenesuflonamides as CCR2 modulators for the treatment of inflammation
Compounds are provided that act as potent antagonists of the CCR2 receptor. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
Triazolyl phenyl benzenesulfonamides
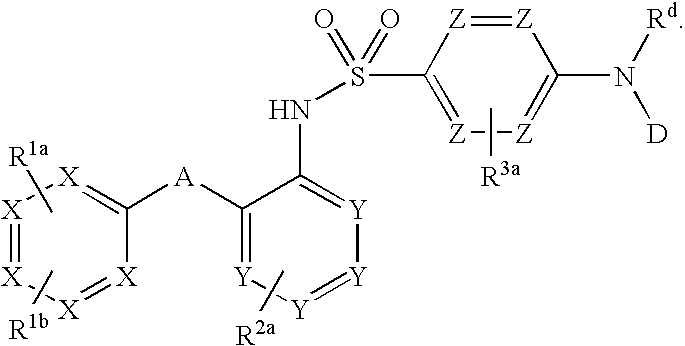
Compounds are provided that act as potent antagonists of the CCR2 or CCR9 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2 and CCR9. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, CCR9-mediated diseases, as controls in assays for the identification of CCR2 antagonists and as controls in assays for the identification of CCR9 antagonists.
Owner:CHEMOCENTRYX INC
Heteroaryl sulfonamides and CCR2/CCR9
Compounds are provided that act as potent antagonists of the CCR2 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
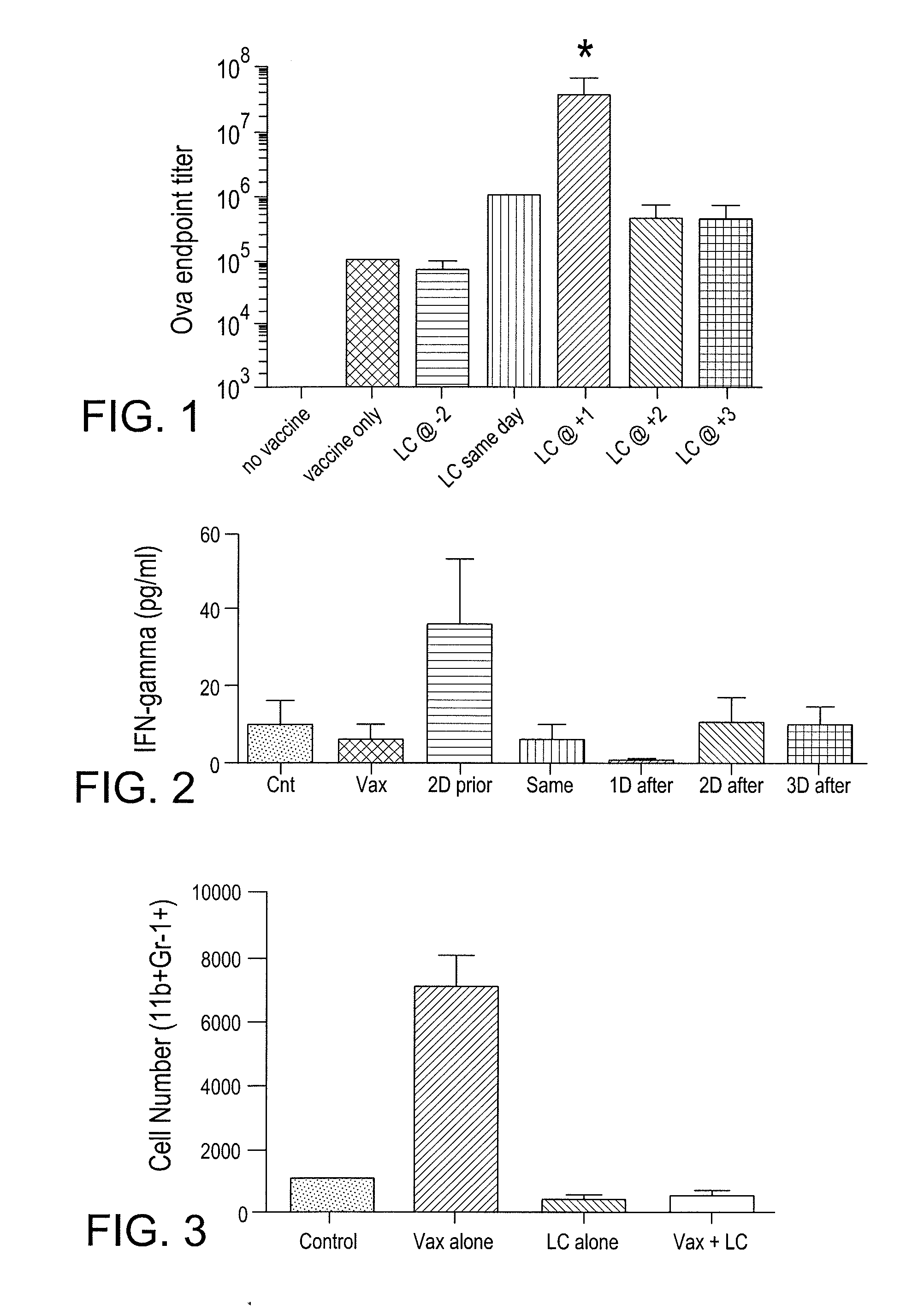
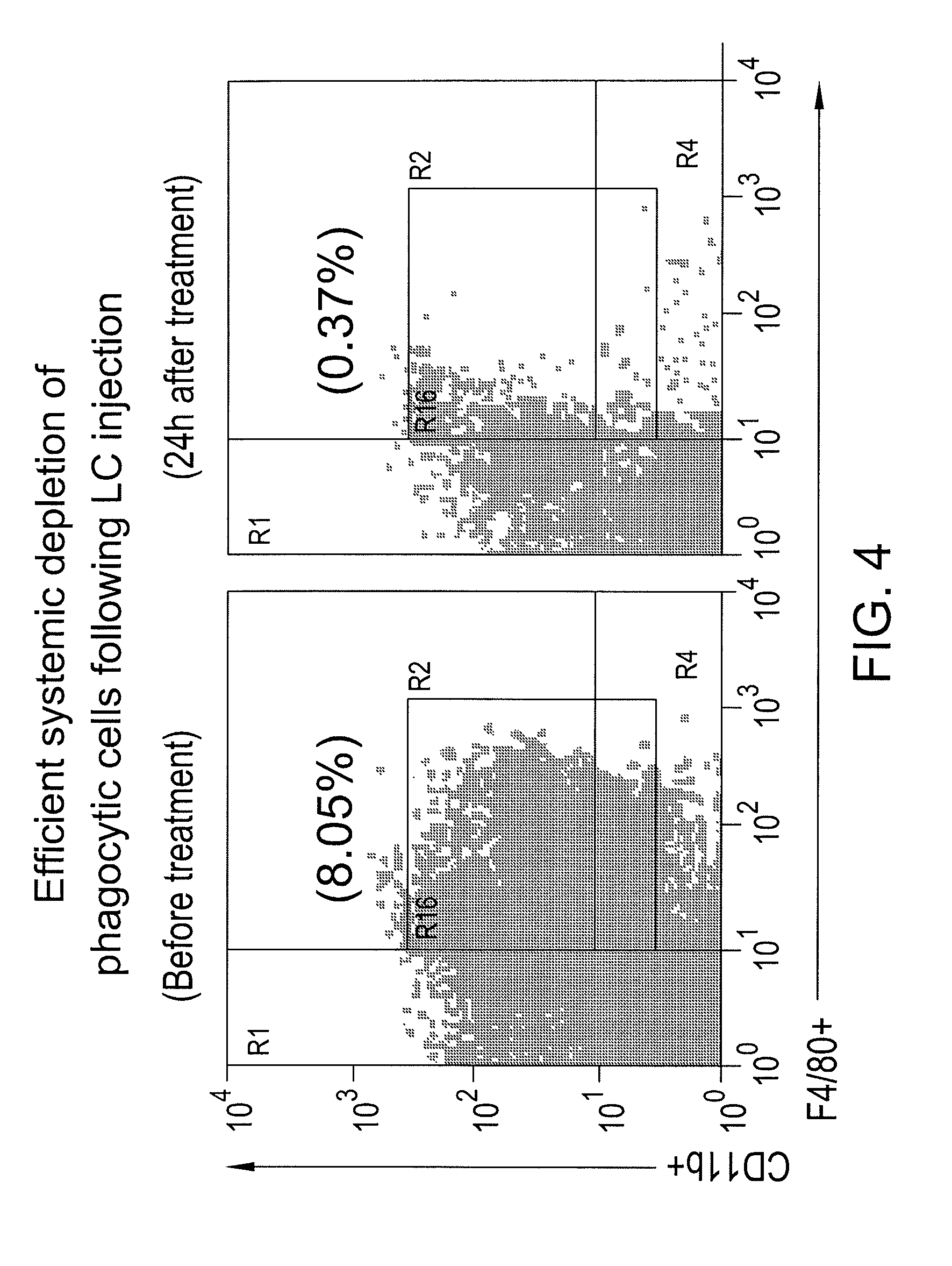
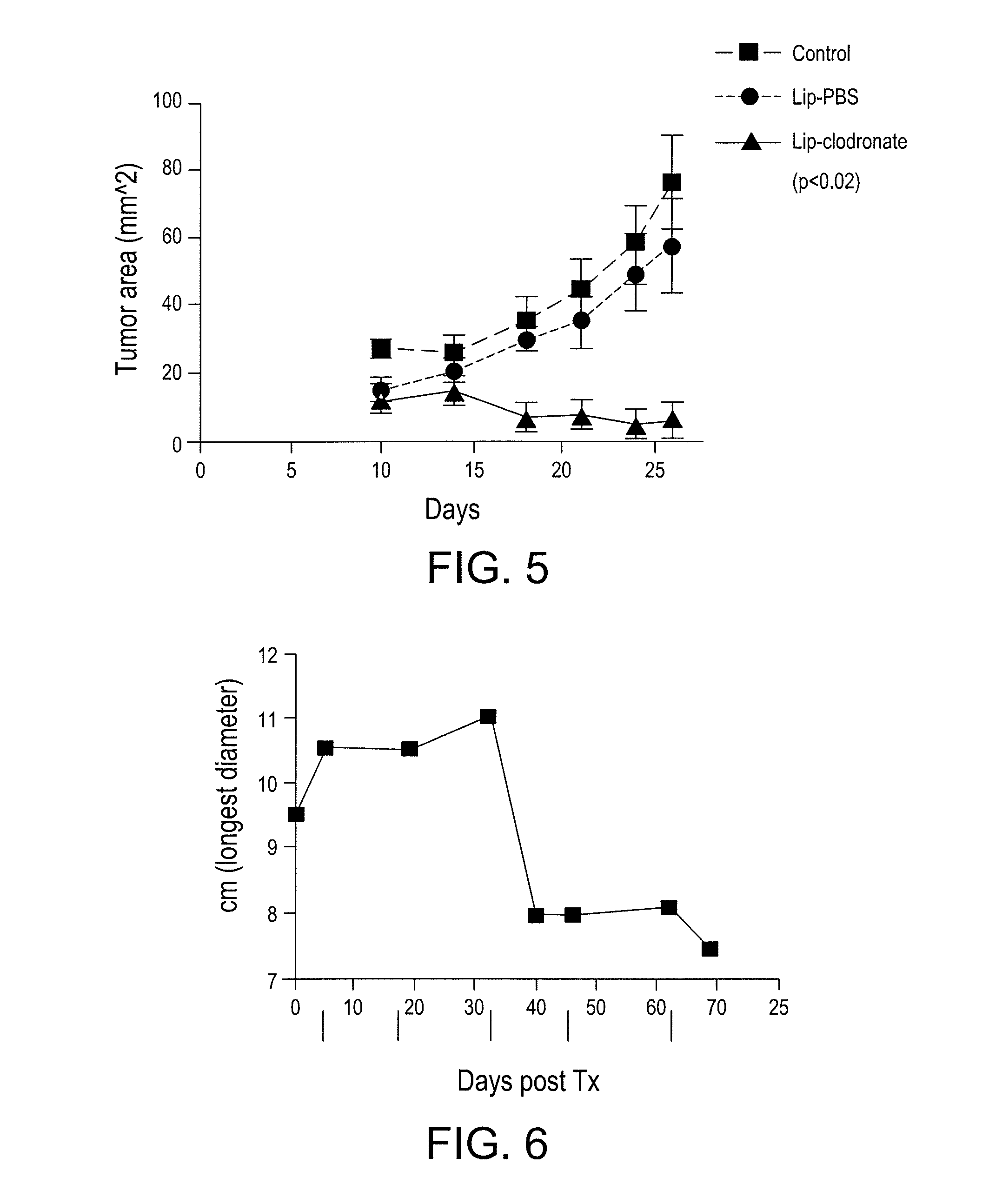
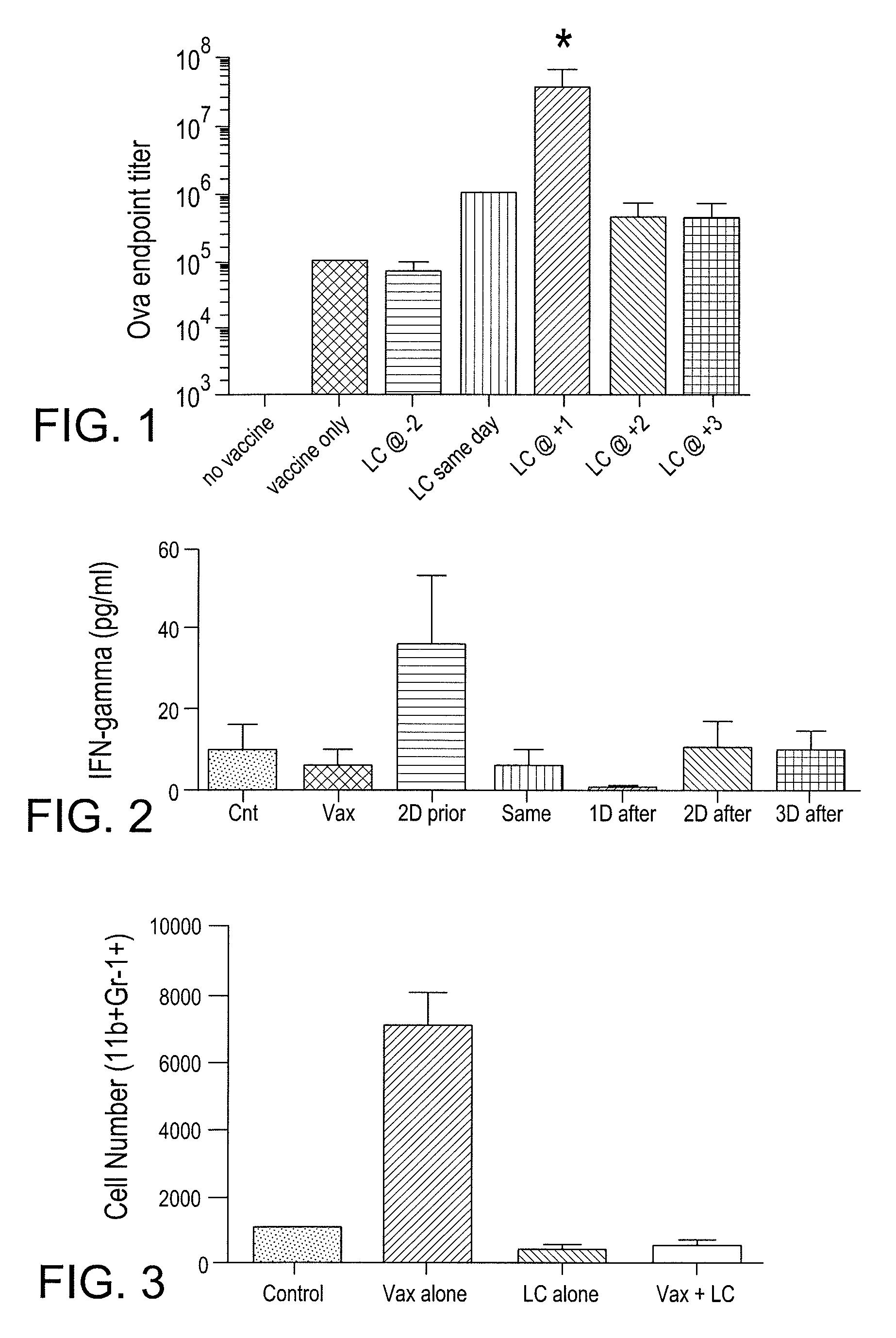
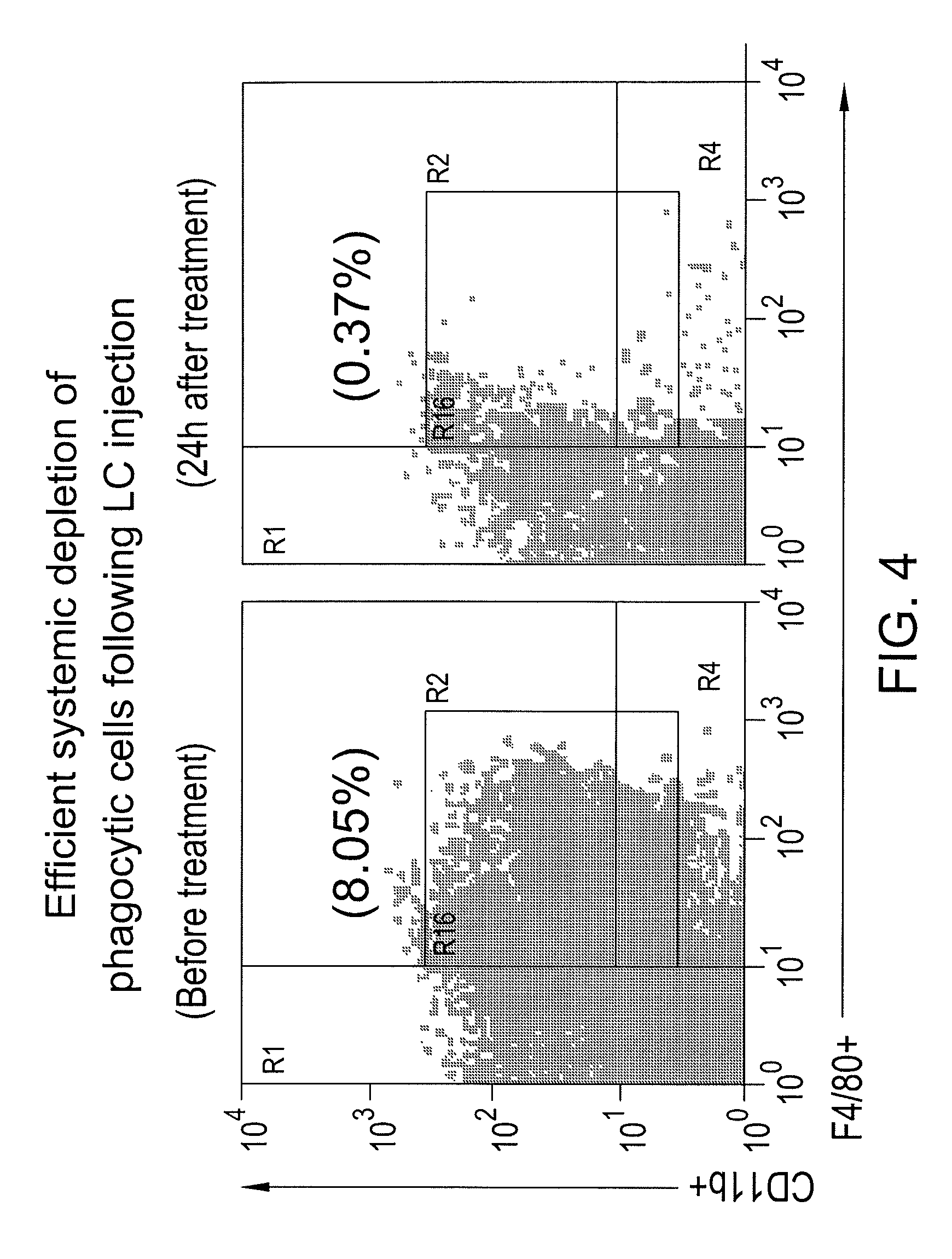
Myeloid derived suppressor cell inhibiting agents
ActiveUS20120156280A1Improve immune activityImproved therapeutic preparationSnake antigen ingredientsAntibody ingredientsAdjuvantCcr2 antagonist
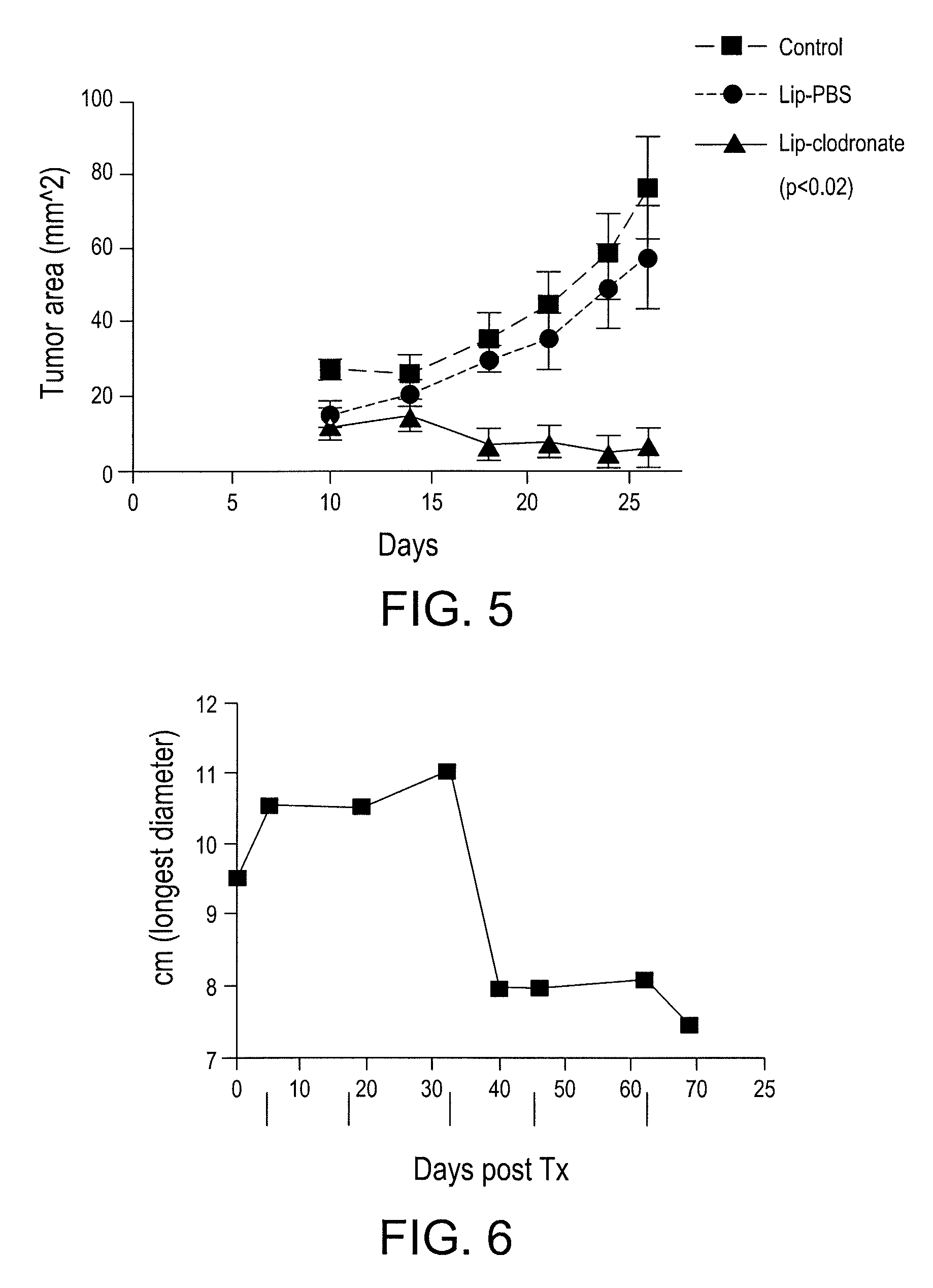
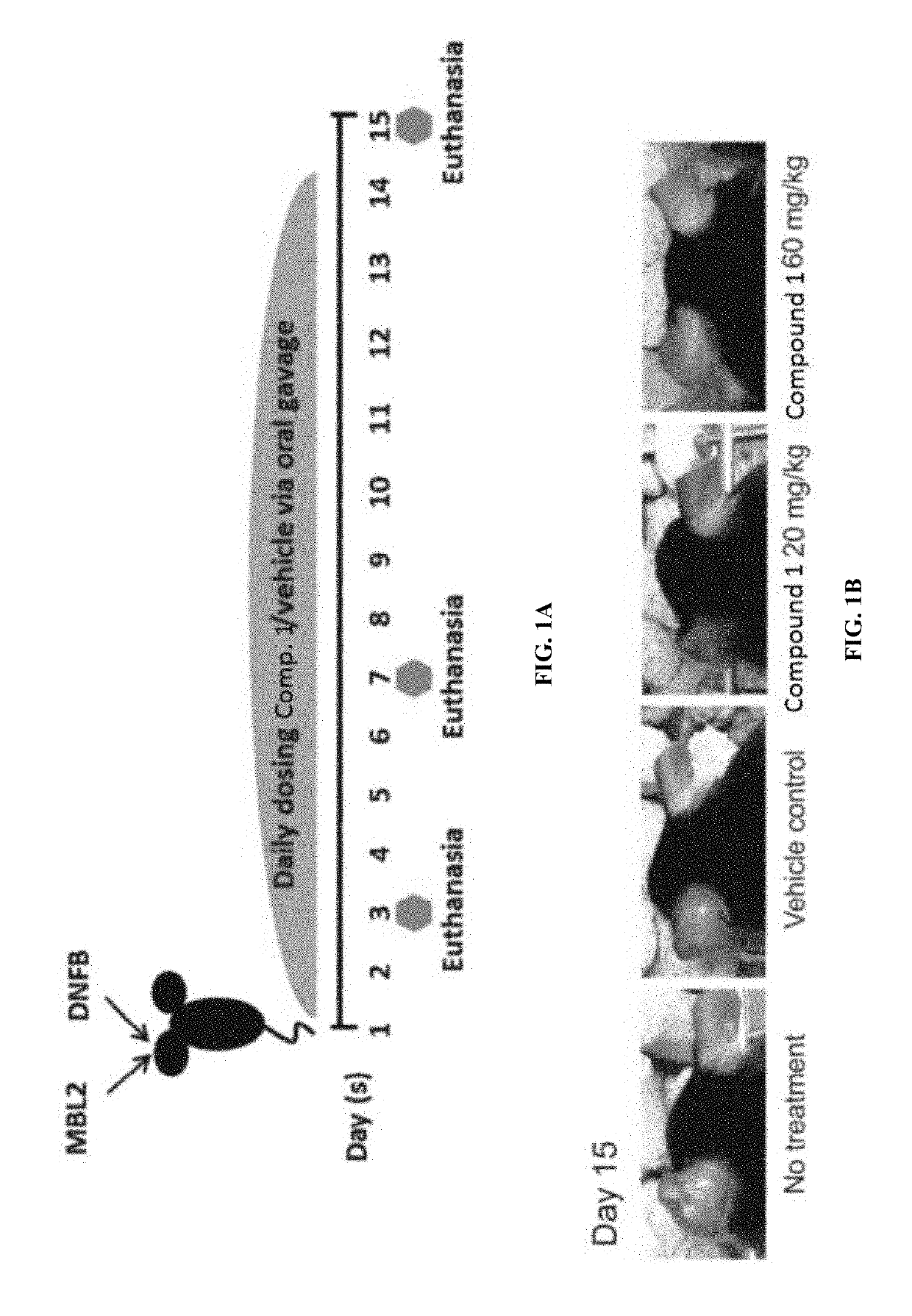
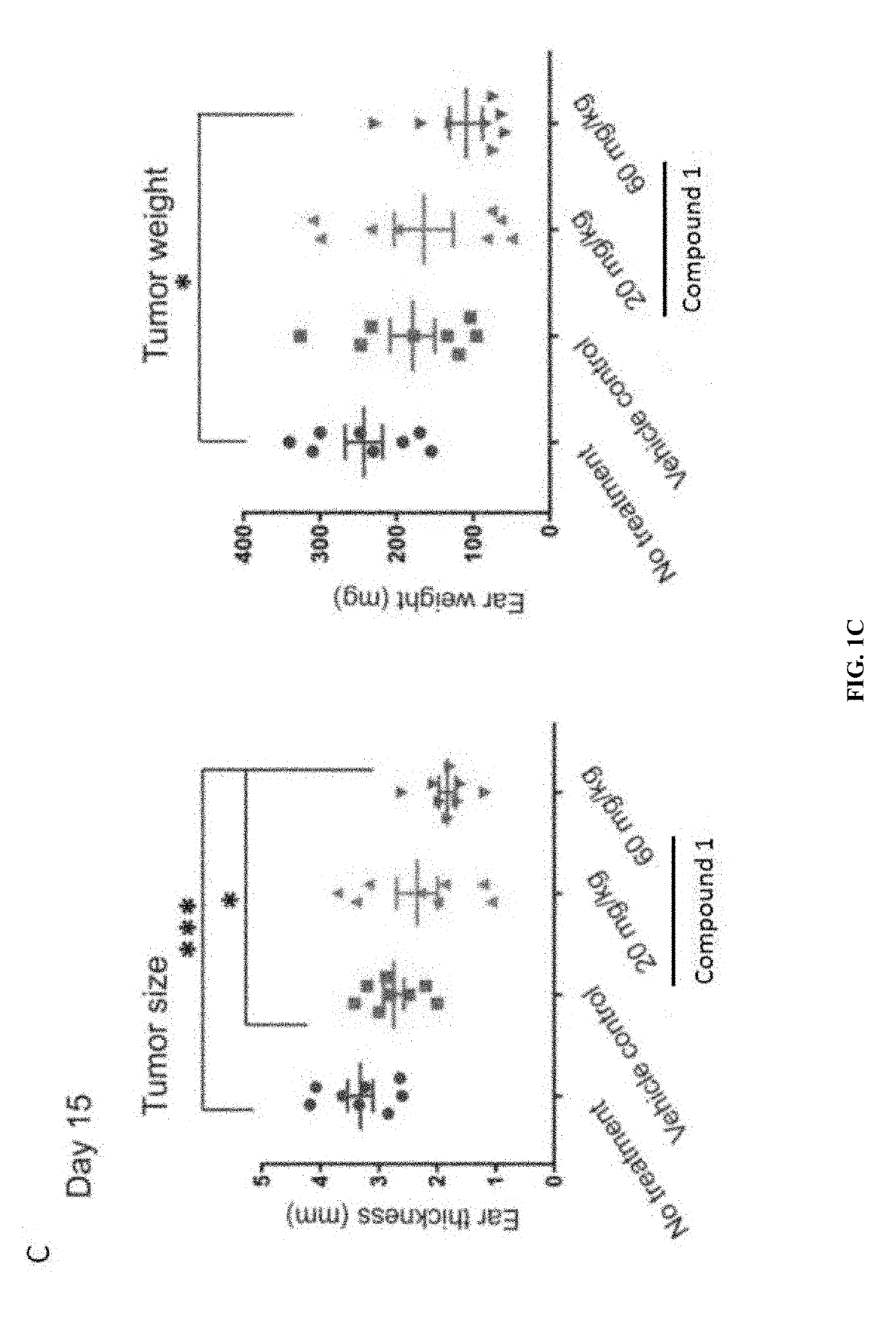
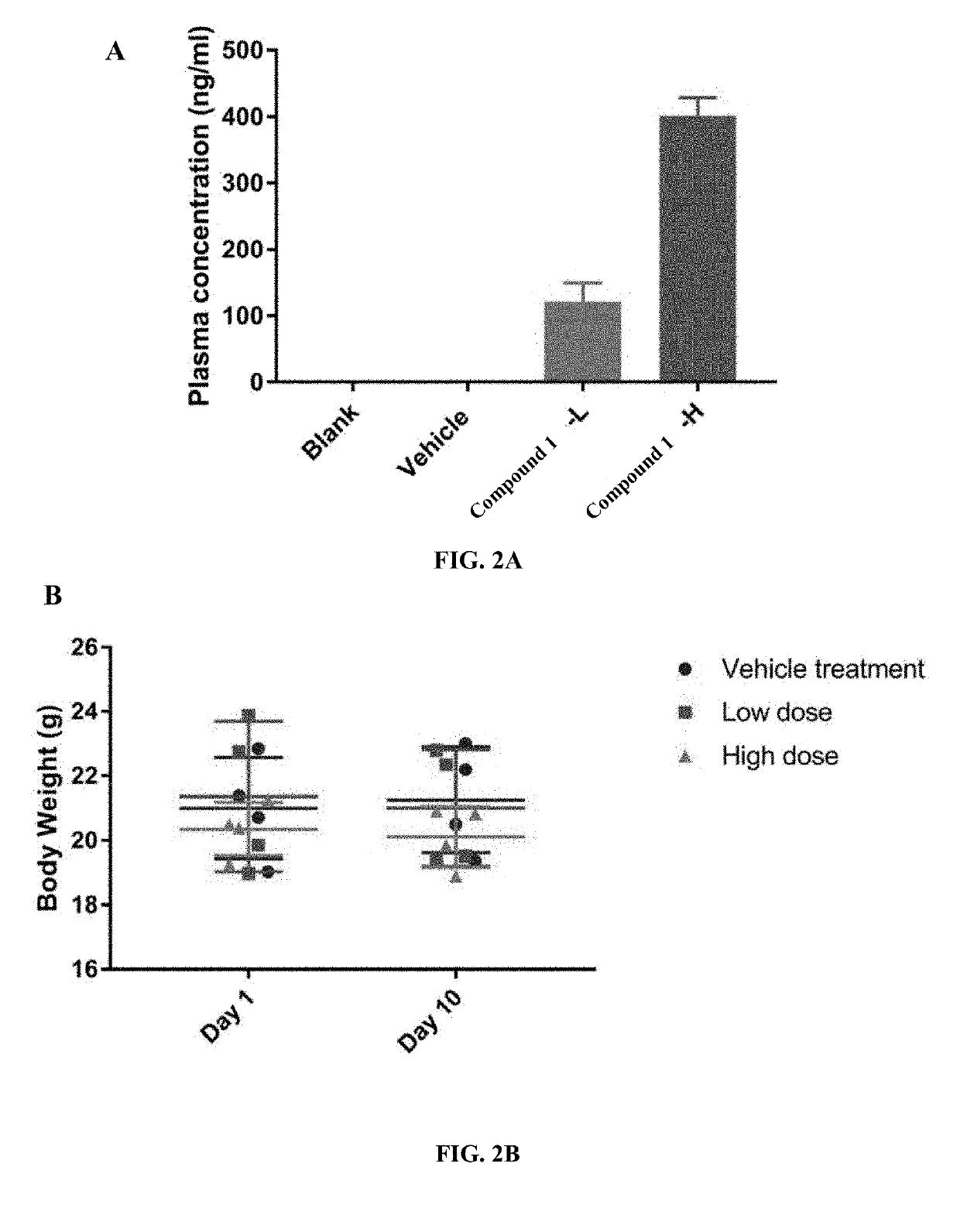
Myeloid derived suppressor cell (MDSC) inhibitory agents and vaccine and / or adjuvant enhancers are provided. Improved vaccine treatment regimens employing these agents are also provided. Cancer vaccines and methods for inhibiting tumor growth and cancer metastases are also presented. The myeloid derived suppressor cell (MDSC) inhibiting agents are described as bisphosphonates (such as liposomal clodronate) and CCR2 inhibitors and / or CCR2 antagonists. Methods for enhancing antibody titer levels in response to an antigen of interest are also provided.
Owner:COLORADO STATE UNIVERSITY
Heteroaryl sulfonamides and ccr2/ccr9
Compounds are provided that act as potent antagonists of the CCR2 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
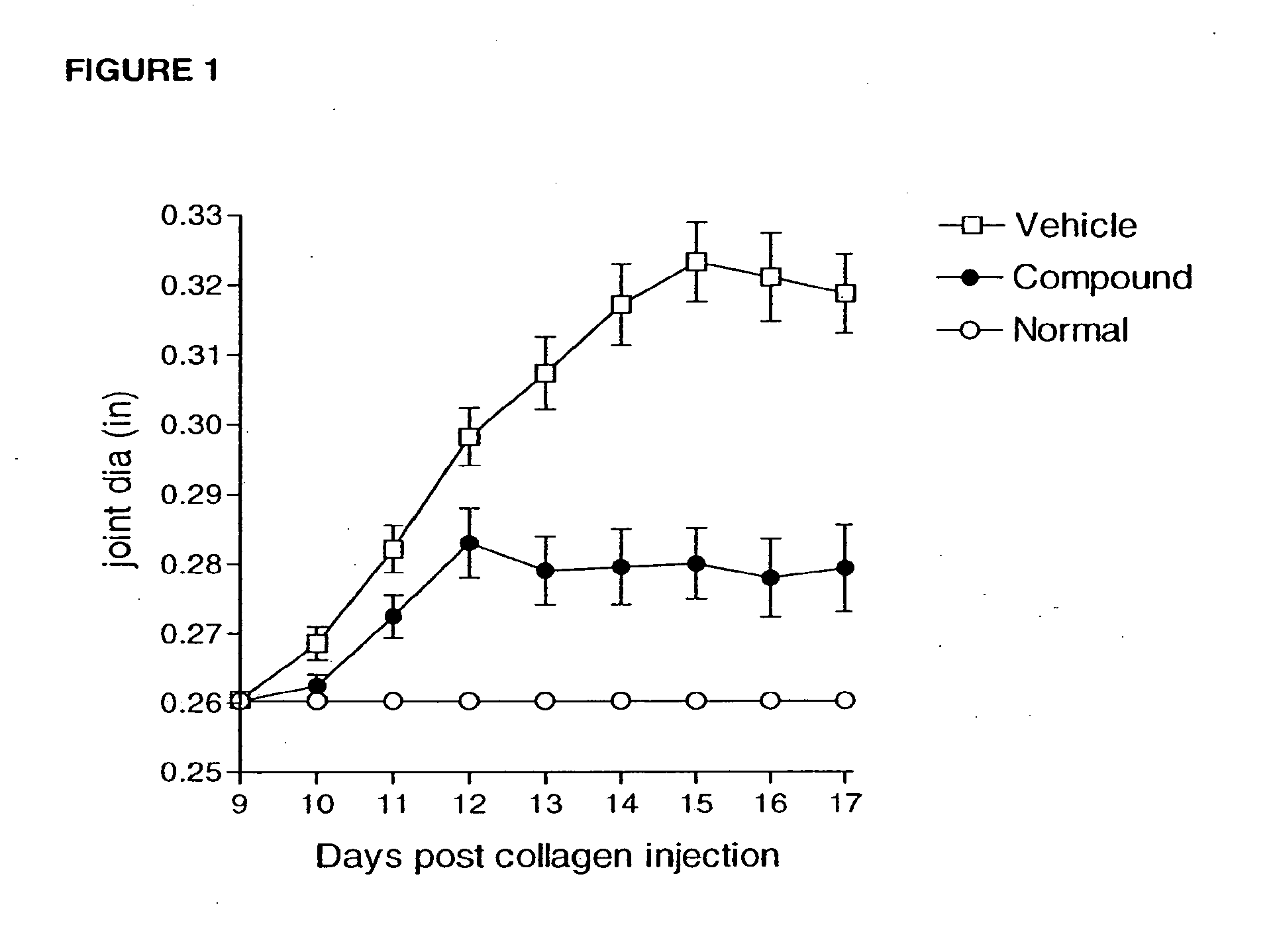
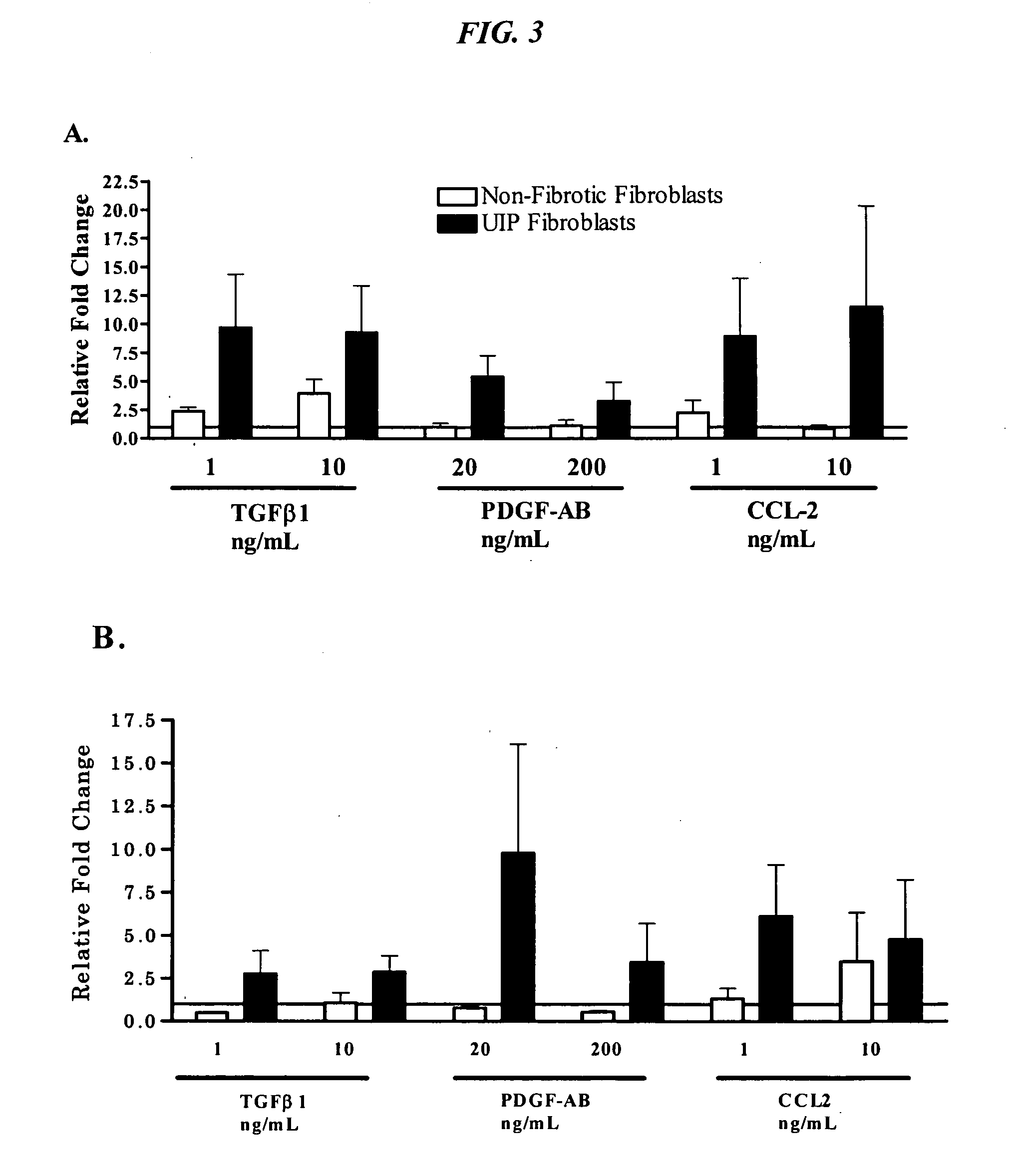
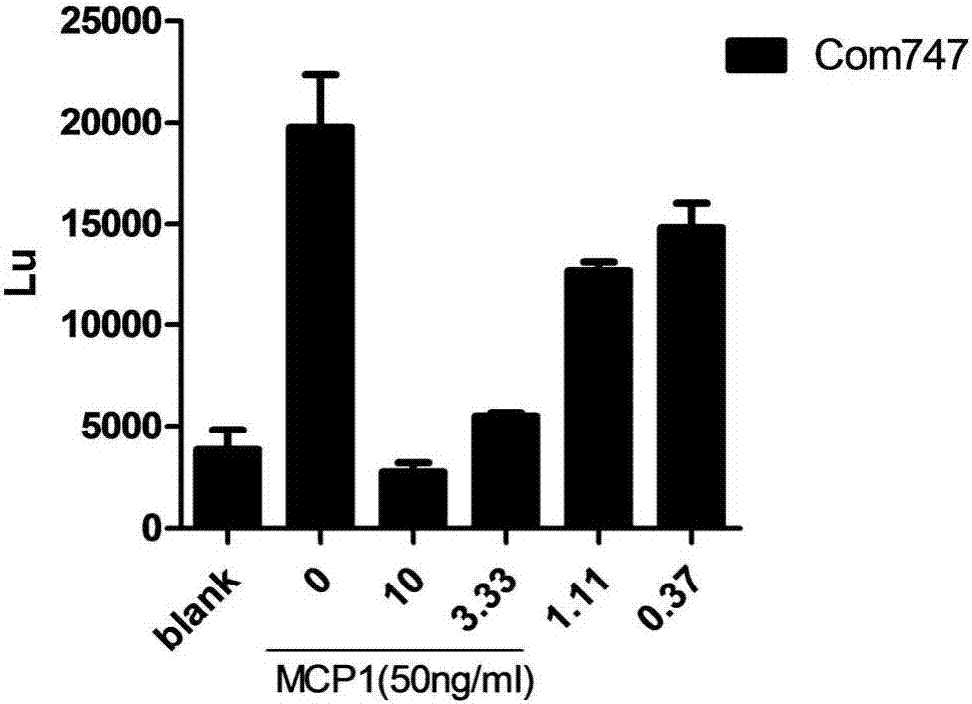
Ccr2 antagonists for treatment of fibrosis
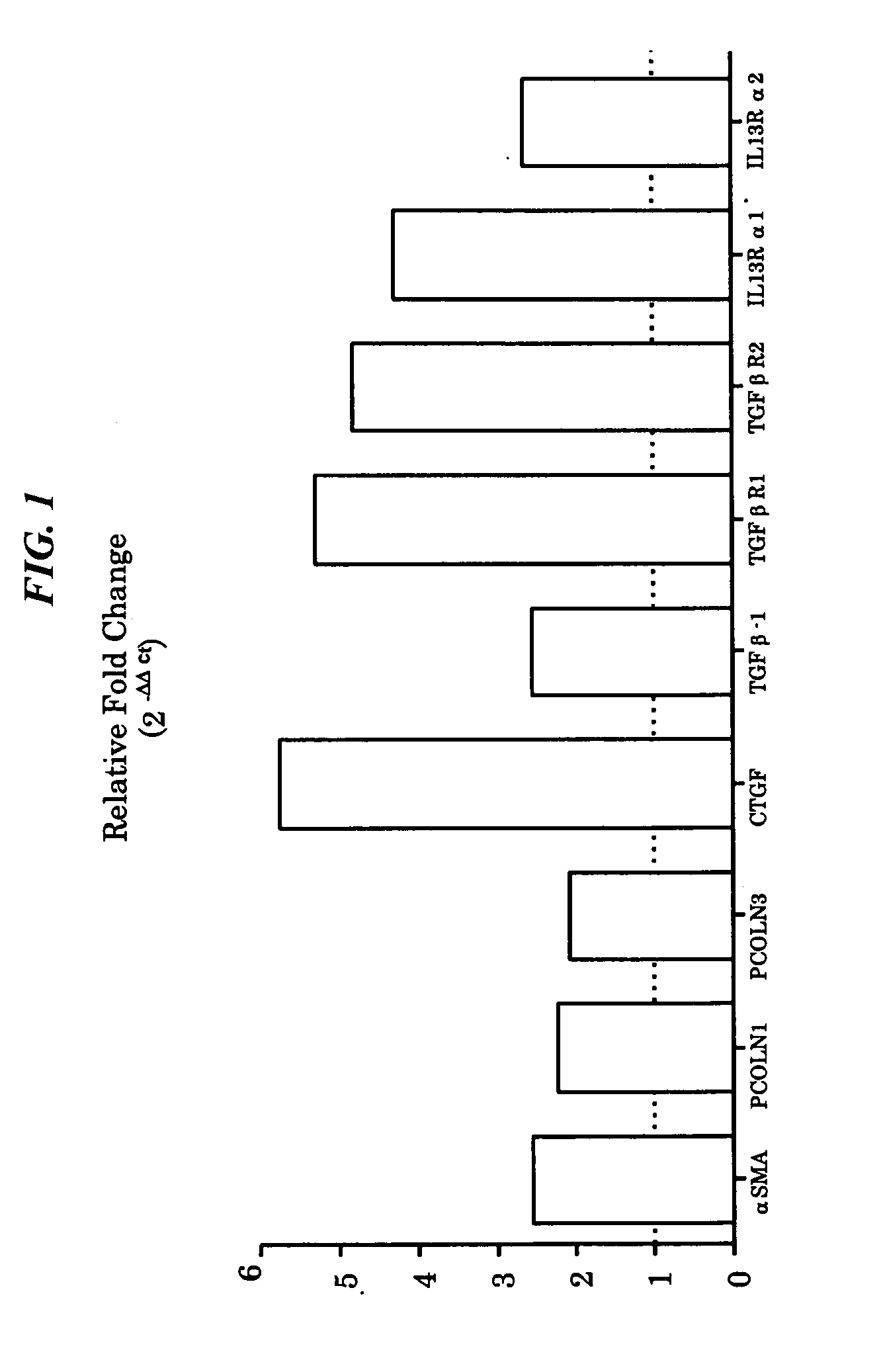
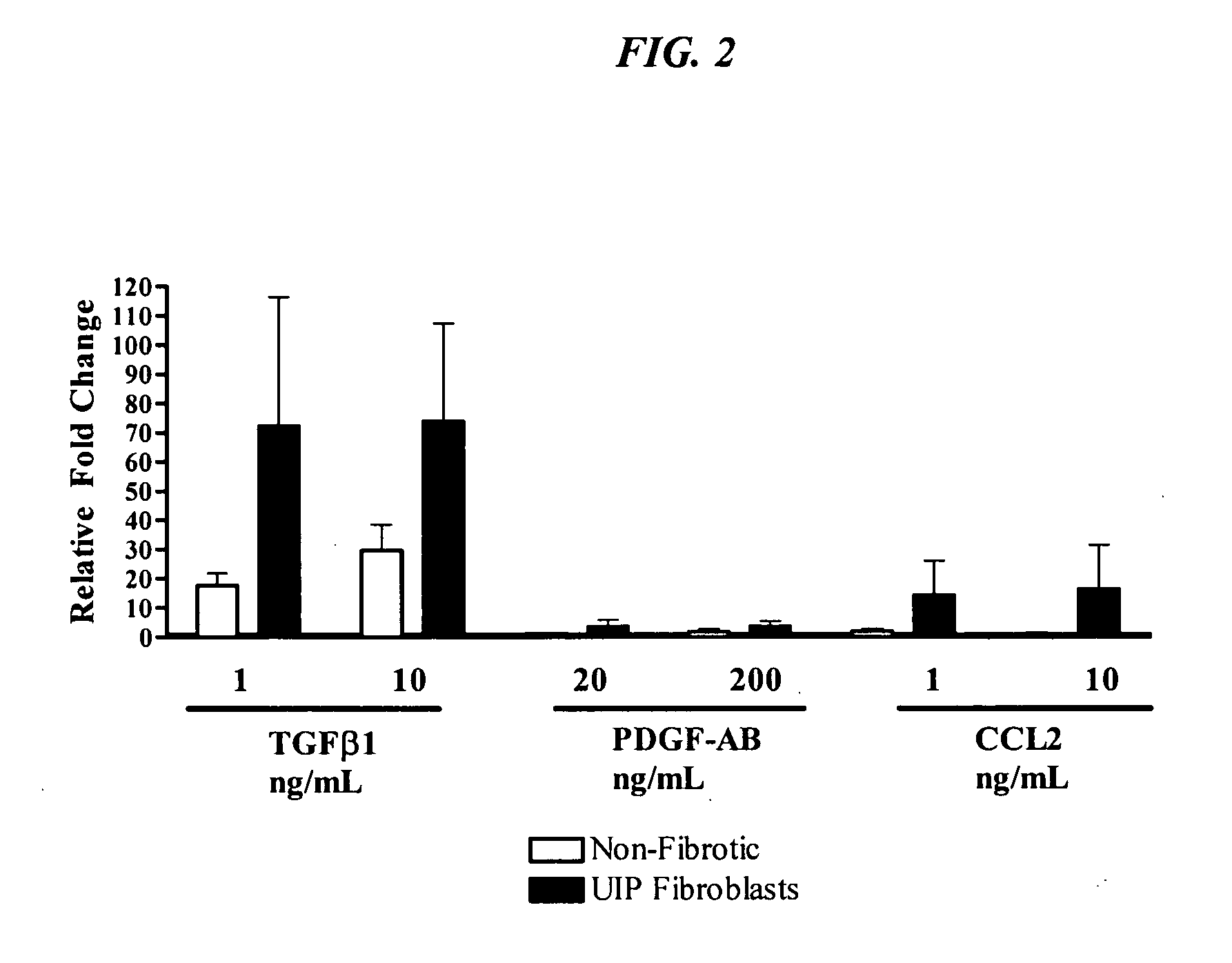
Anti-MCP-1 / CCR2 antagonist therapy is provided for the control or reversal of fibrosis related diseases, including, e.g., but not limited to MCP-1 / CCR2 antagonist therapy for the modulation of profibrotic markers associated with fibrotic processes including collagen matrix deposition and alveolar collapse.
Owner:CENTOCOR ORTHO BIOTECH
Ccr2 inhibitors and methods of use thereof
InactiveUS20100234364A1Modulating chemokine activityModulating chemokine functionBiocideOrganic chemistryArylDisease
Compounds are provided that act as potent antagonists of the CCR2 or CCR9 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2 and CCR9. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, CCR9-mediated diseases, as controls in assays for the identification of CCR2 antagonists, and as controls in assays for the identification of CCR9 antagonists.
Owner:BASAK ARINDRAJIT +6
Triazolyl phenyl benzenesulfonamides
ActiveUS20080039465A1Modulating chemokine activityModulating chemokine functionBiocideNervous disorderDiseaseAryl
Compounds are provided that act as potent antagonists of the CCR2 or CCR9 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2 and CCR9. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, CCR9-mediated diseases, as controls in assays for the identification of CCR2 antagonists and as controls in assays for the identification of CCR9 antagonists.
Owner:CHEMOCENTRYX INC
Triazolyl pyridyl benzenesulfonamides
Compounds are provided that act as potent antagonists of the CCR2 or CCR9 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2 and CCR9. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, CCR9-mediated diseases, as controls in assays for the identification of CCR2 antagonists and as controls in assays for the identification of CCR9 antagonists.
Owner:CHEMOCENTRYX INC
Fused heteroaryl pyridyl and phenyl benzenesuflonamides as ccr2 modulators for the treatment of inflammation
ActiveUS20090233946A1Modulating chemokine activityModulating chemokine functionAntibacterial agentsBiocideDiseaseAryl
Compounds are provided that act as potent antagonists of the CCR2 receptor. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases and as controls in assays for the identification of CCR2 antagonists.
Owner:CHEMOCENTRYX INC
Triazolyl phenyl benzenesulfonamides
Compounds are provided that act as potent antagonists of the CCR2 or CCR9 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2 and CCR9. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, CCR9-mediated diseases, as controls in assays for the identification of CCR2 antagonists and as controls in assays for the identification of CCR9 antagonists.
Owner:CHEMOCENTRYX INC
Triazolyl pyridyl benzenesulfonamides
Compounds are provided that act as potent antagonists of the CCR2 or CCR9 receptor. Animal testing demonstrates that these compounds are useful for treating inflammation, a hallmark disease for CCR2 and CCR9. The compounds are generally aryl sulfonamide derivatives and are useful in pharmaceutical compositions, methods for the treatment of CCR2-mediated diseases, CCR9-mediated diseases, as controls in assays for the identification of CCR2 antagonists and as controls in assays for the identification of CCR9 antagonists.
Owner:CHEMOCENTRYX INC
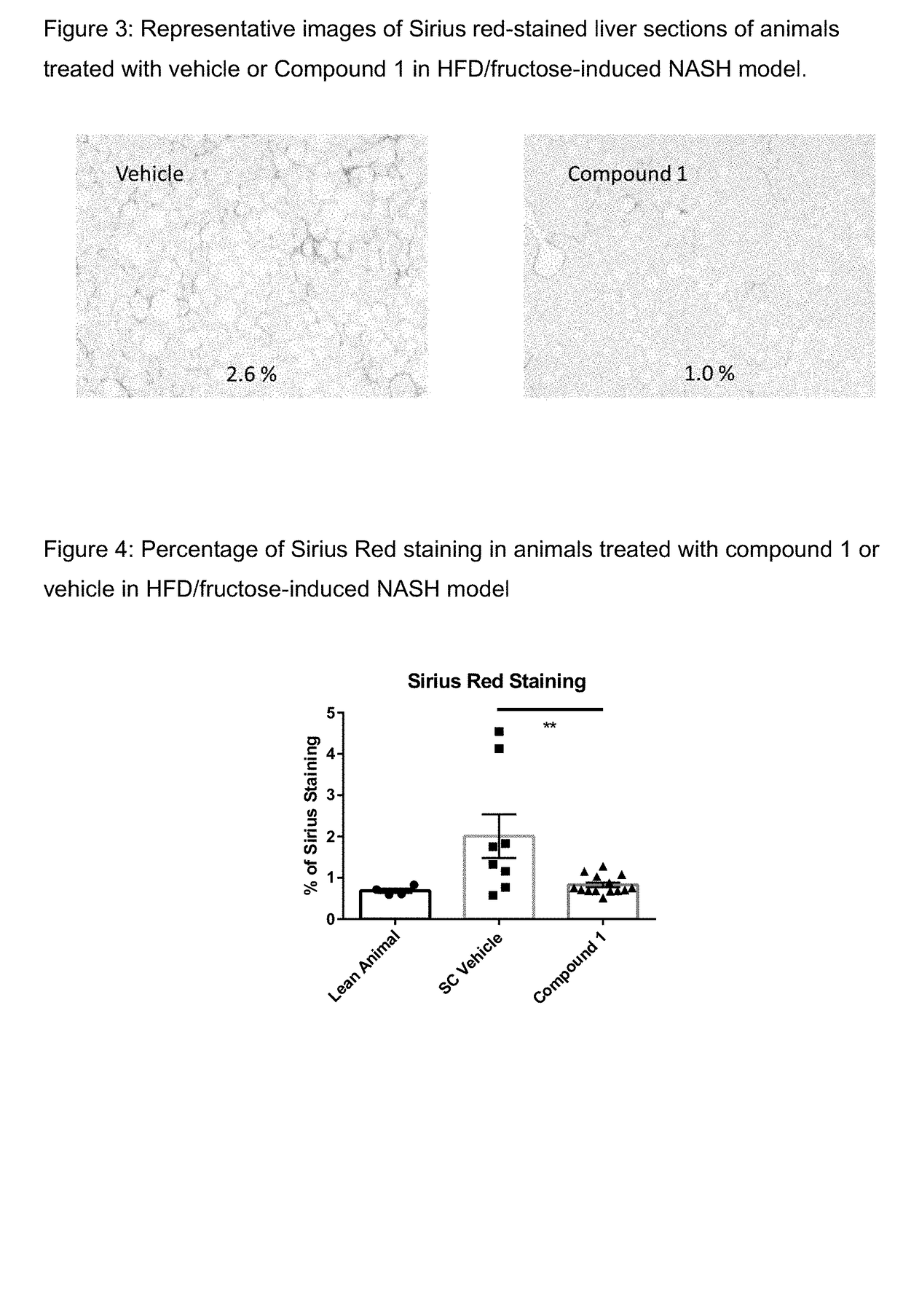
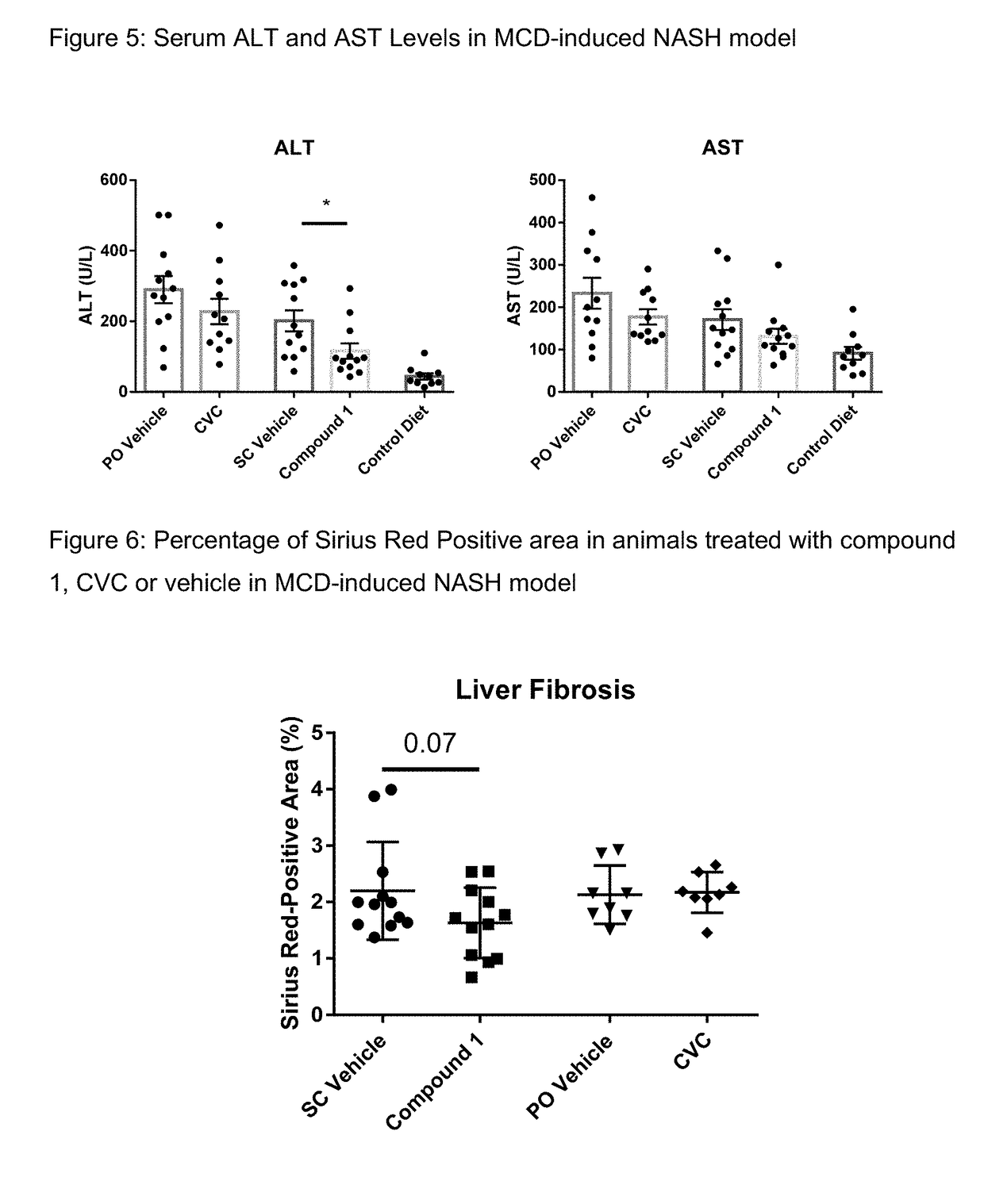
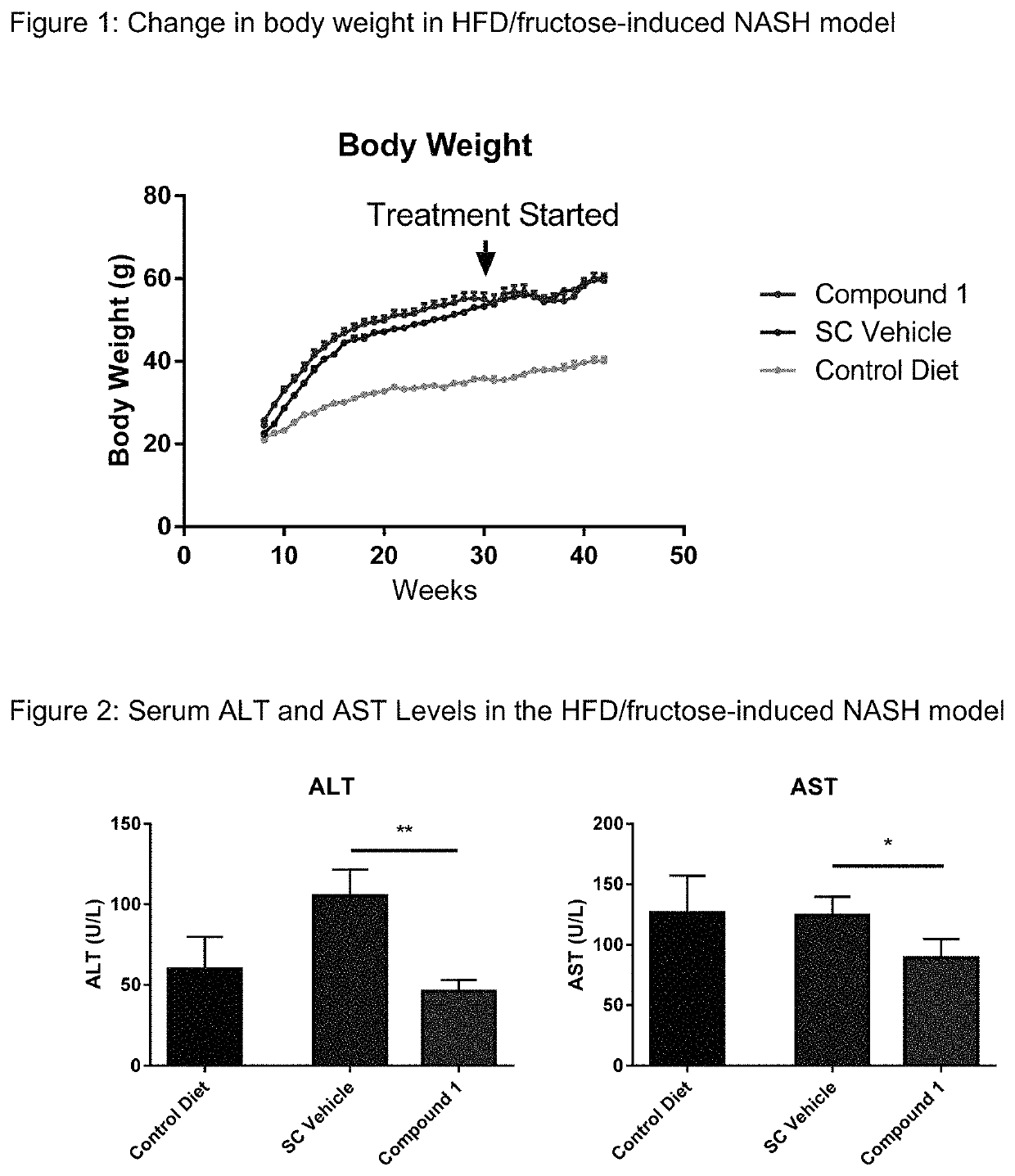
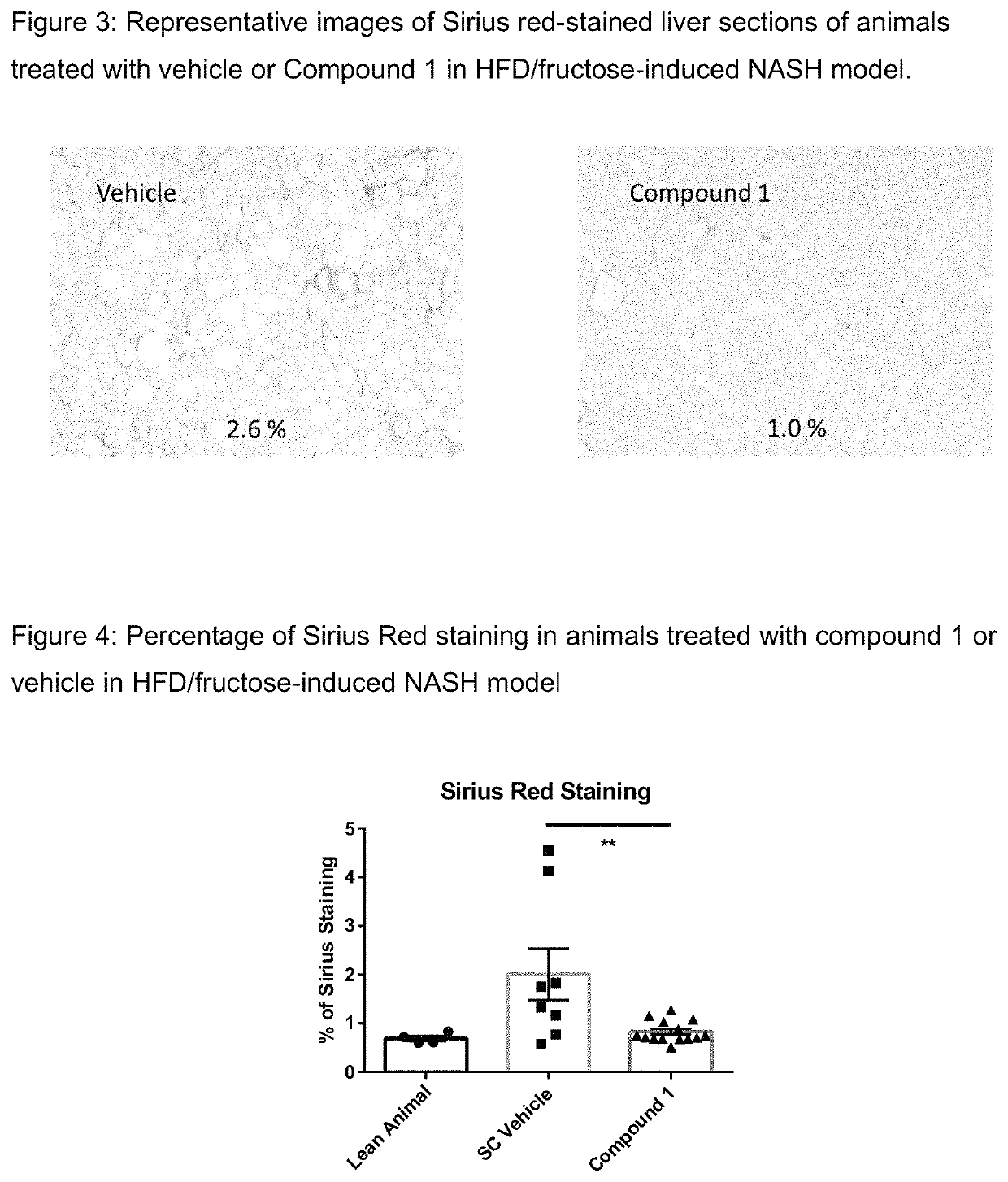
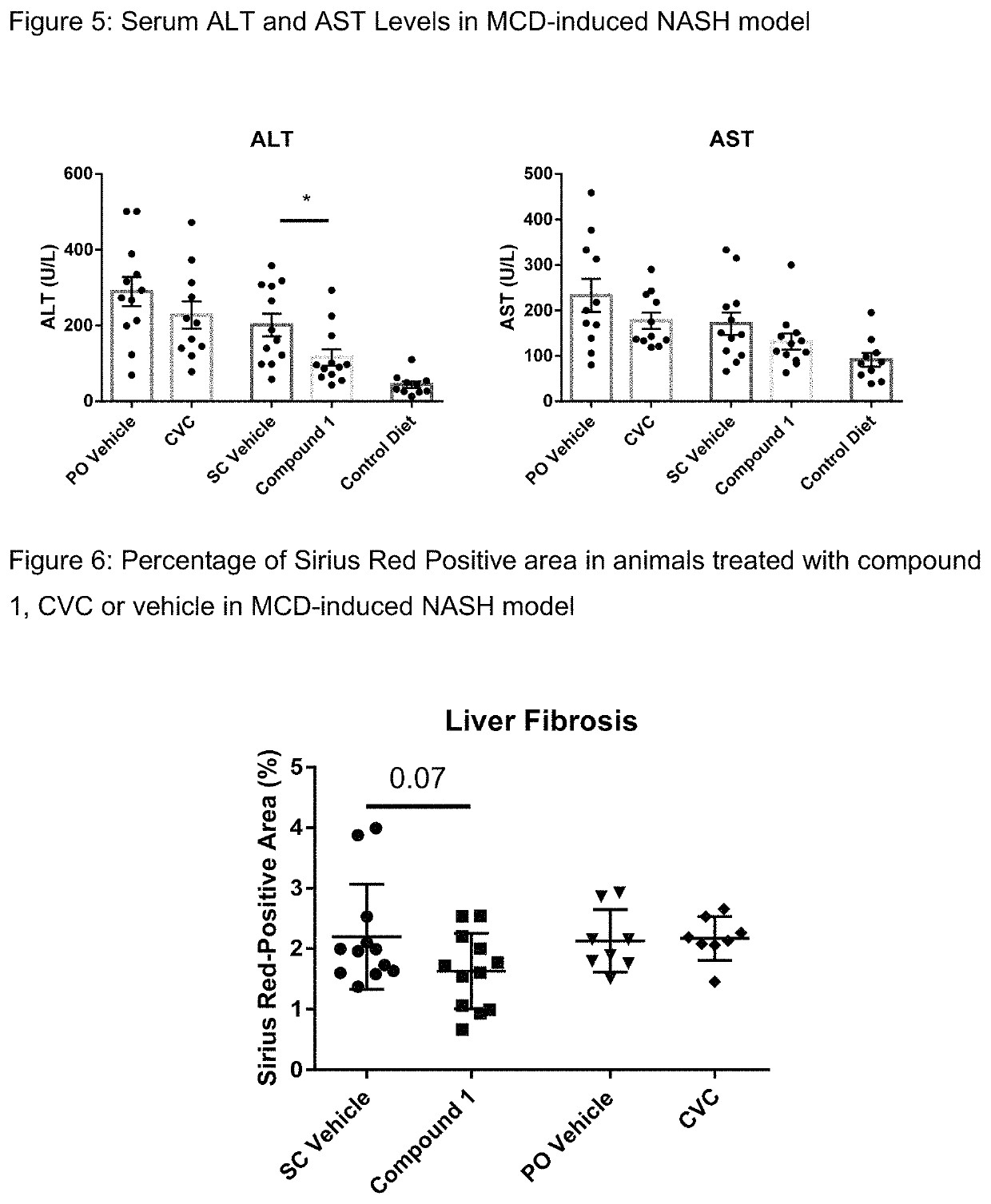
Method of treating liver fibrosis
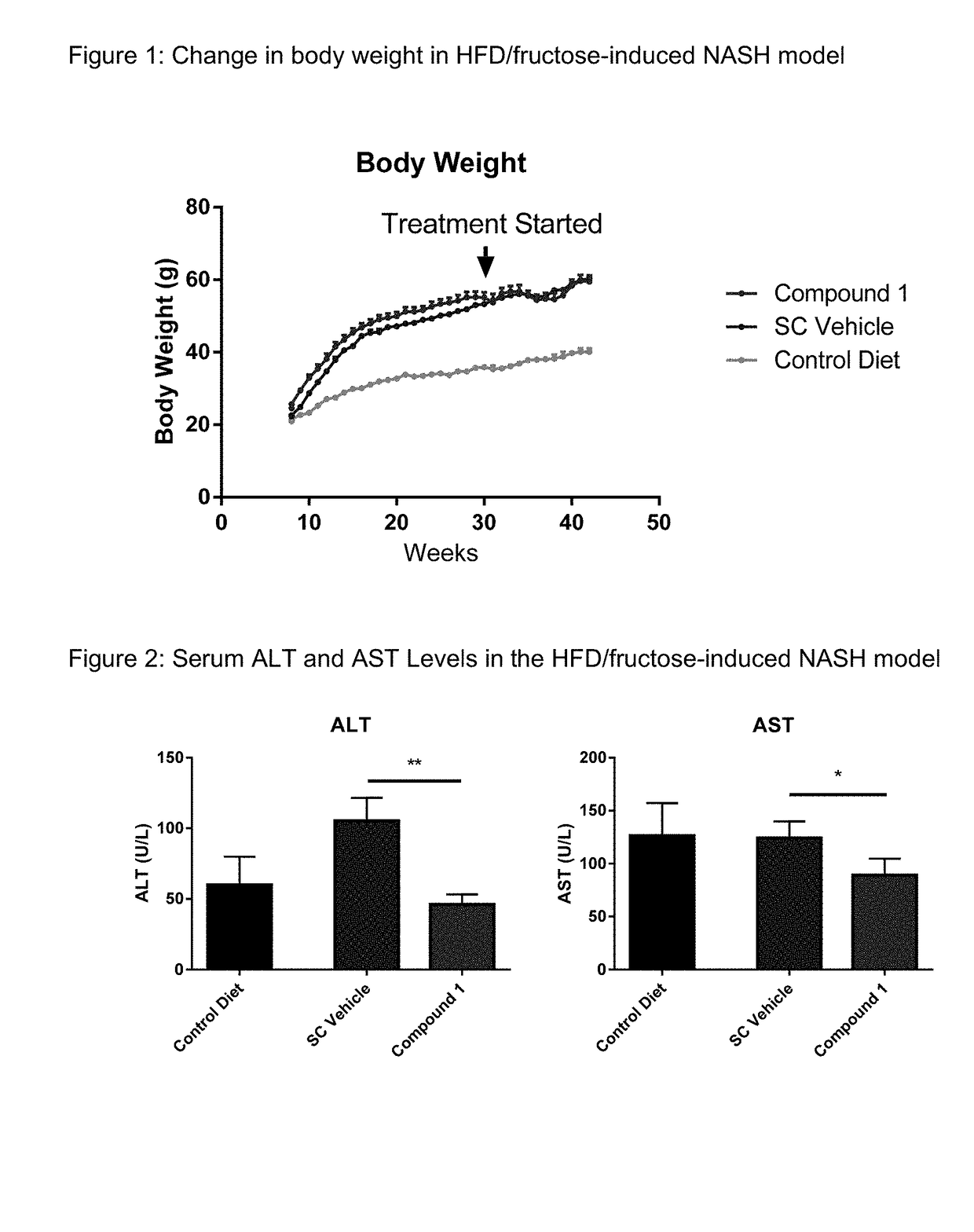
A method of treating liver fibrosis with CCR2 antagonists is provided. The liver fibrosis may be associated with non-alcoholic steatohepatitis (NASH), non-alcoholic fatty liver disease (NAFLD), emerging cirrhosis, non-cirrhotic hepatic fibrosis, type 2 diabetes mellitus (T2DM) or metabolic syndrome (MS).
Owner:CHEMOCENTRYX INC
Myeloid derived suppressor cell inhibiting agents
ActiveUS9320735B2Improve immune activityImprove efficiencySnake antigen ingredientsAntibody ingredientsAdjuvantCancer metastasis
Myeloid derived suppressor cell (MDSC) inhibitory agents and vaccine and / or adjuvant enhancers are provided. Improved vaccine treatment regimens employing these agents are also provided. Cancer vaccines and methods for inhibiting tumor growth and cancer metastases are also presented. The myeloid derived suppressor cell (MDSC) inhibiting agents are described as bisphosphonates (such as liposomal clodronate) and CCR2 inhibitors and / or CCR2 antagonists. Methods for enhancing antibody titer levels in response to an antigen of interest are also provided.
Owner:COLORADO STATE UNIVERSITY
Methods of treating solid tumors with CCR2 antagonists
InactiveUS20190269664A1Reduce in quantityOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCcr2 antagonistCD8
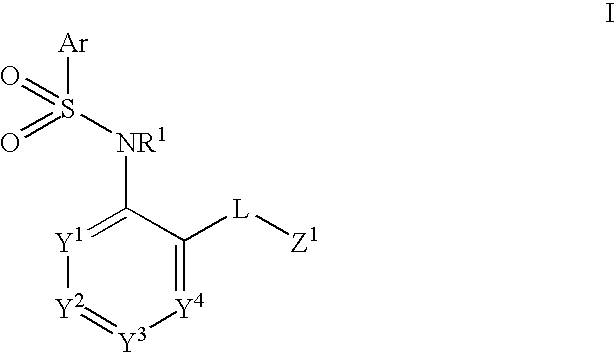
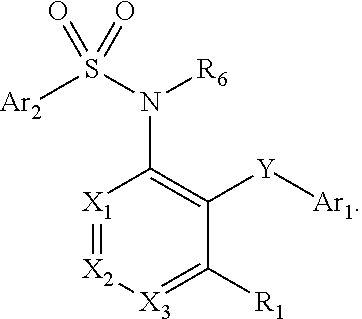
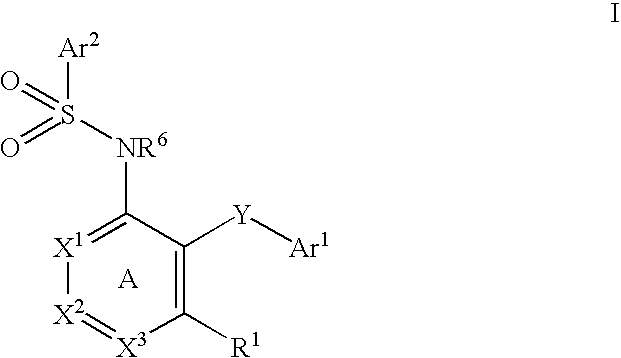
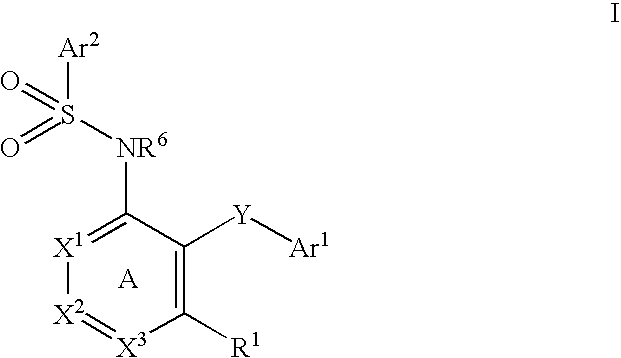
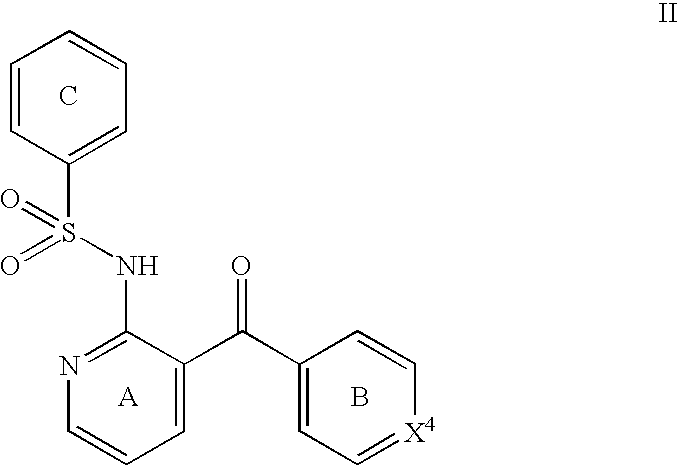
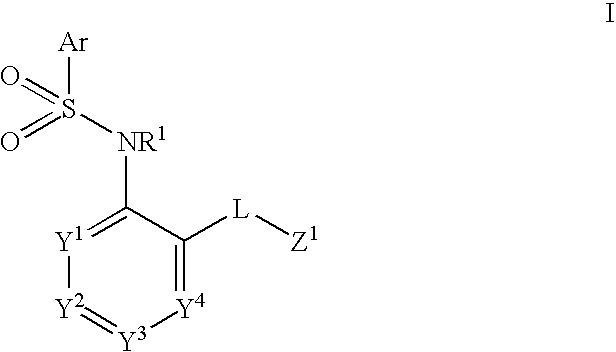
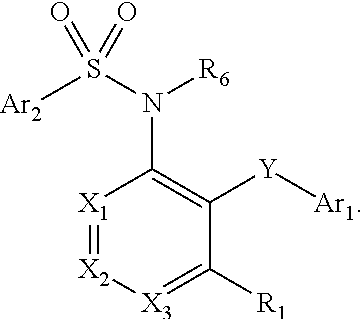
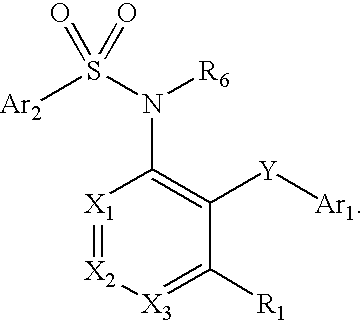
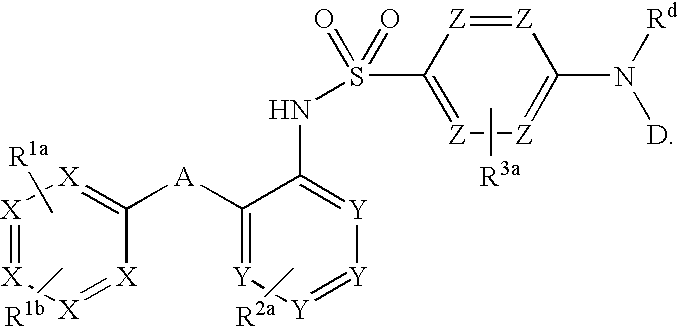
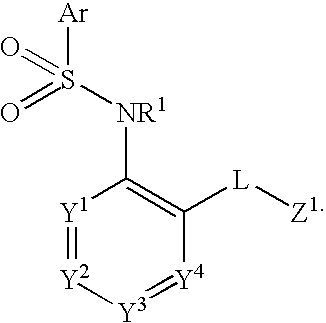
The present disclosure provides, inter alia, methods of treating a solid-tumor by administering an effective amount of a Chemokine Receptor 2 (CCR2) antagonist. Also provided herein are methods of reducing the number of macrophages in a solid tumor microenvironment, said method comprising administering effective amount of a Chemokine Receptor 2 (CCR2) antagonist. In an additional aspect, the current disclosure further provides methods of increasing the number CD8+ T cells in a solid tumor microenvironment, said method comprising administering effective amount of a Chemokine Receptor 2 (CCR2) antagonist. In some embodiments, the CCR2 antagonist has the formula I or Formula III:
Owner:RGT UNIV OF CALIFORNIA +1
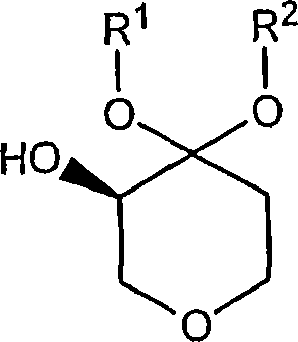
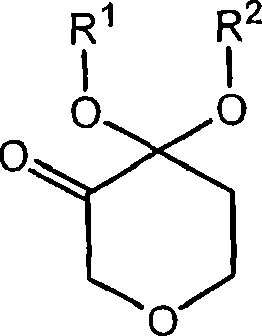
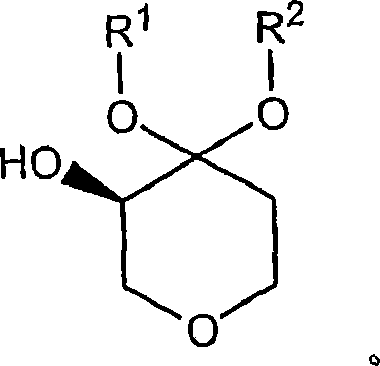
Process for the preparation of (r)-4,4-dialkoxy-pyran-3-ols such as (r)-4,4-dimethoxy-pyran-3-ol
The present invention is concerned with novel processes for the preparation of (R)-4,4-dimethoxy-pyran-3-ol. This compound is useful as an intermediate in the synthesis of compounds which possess pharmacological activity including CCR2 antagonists.
Owner:MERCK & CO INC
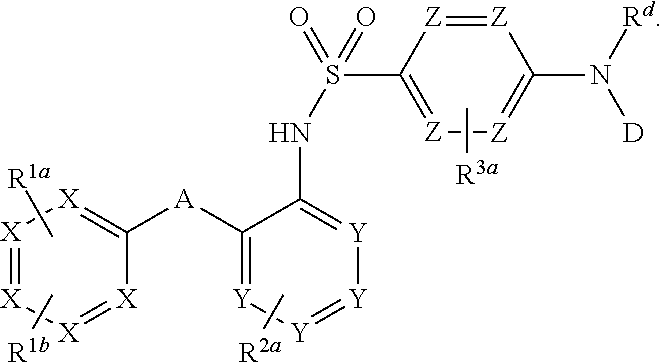
Novel CCR2 antagonist and application thereof
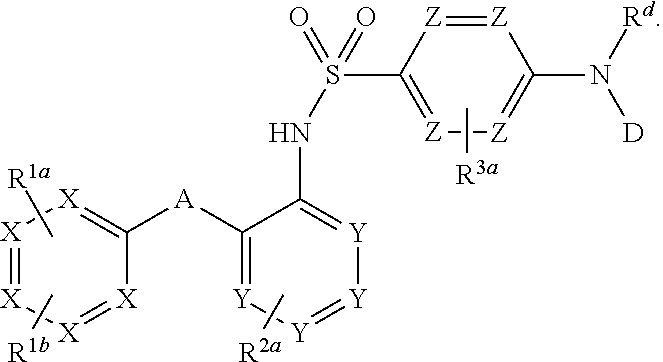
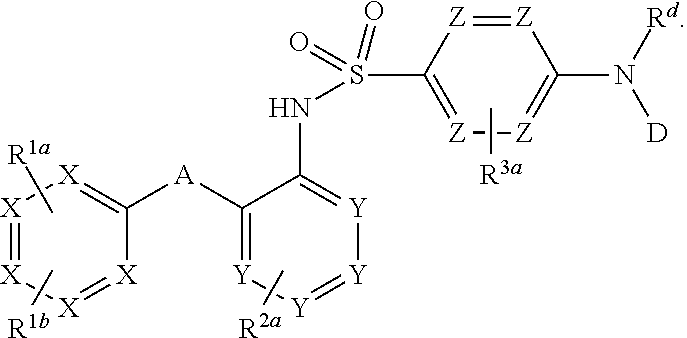
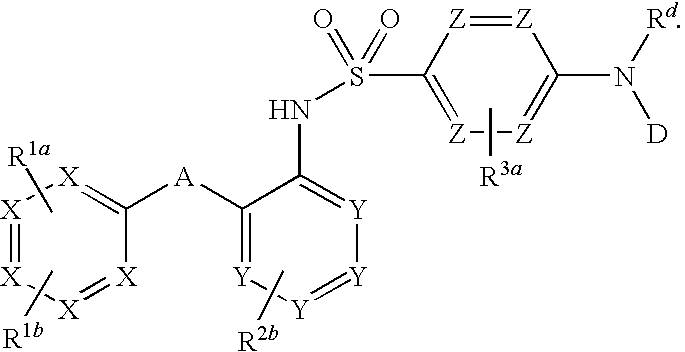
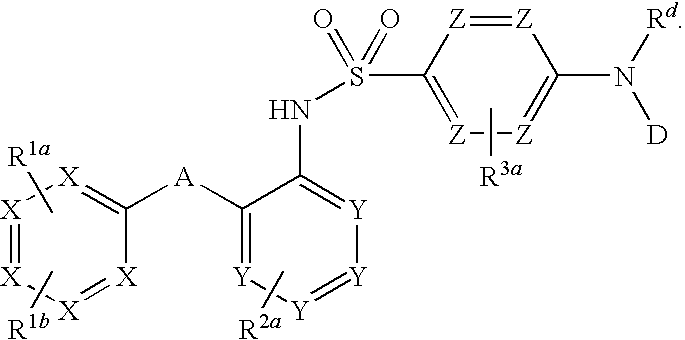
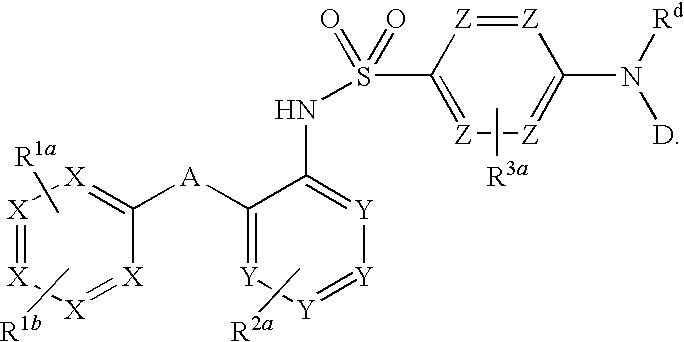
The invention relates to a novel CCR2 antagonist and an application thereof. Specifically, the invention discloses an application of a compound as shown in formula I (referring to the Description) or pharmaceutically acceptable salt in preparing medicines or reagents for regulating tumor microenvironment, relieving aggregation of macrophage or bone marrow cells towards primary tumors, inhibiting tumor growth, inhibiting angiogenesis and / or increasing CD8T cells in a required object, as well as an application in preparing medicines for preventing or treating CCR2-mediated diseases in the object or an application in preparing medicines or reagents for inhibiting a CCR2 or CCL2-CCR2 pathway, wherein in the formula, a, b, c, d, R1, R2, R3 and R4 are as described in the Description.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI +1
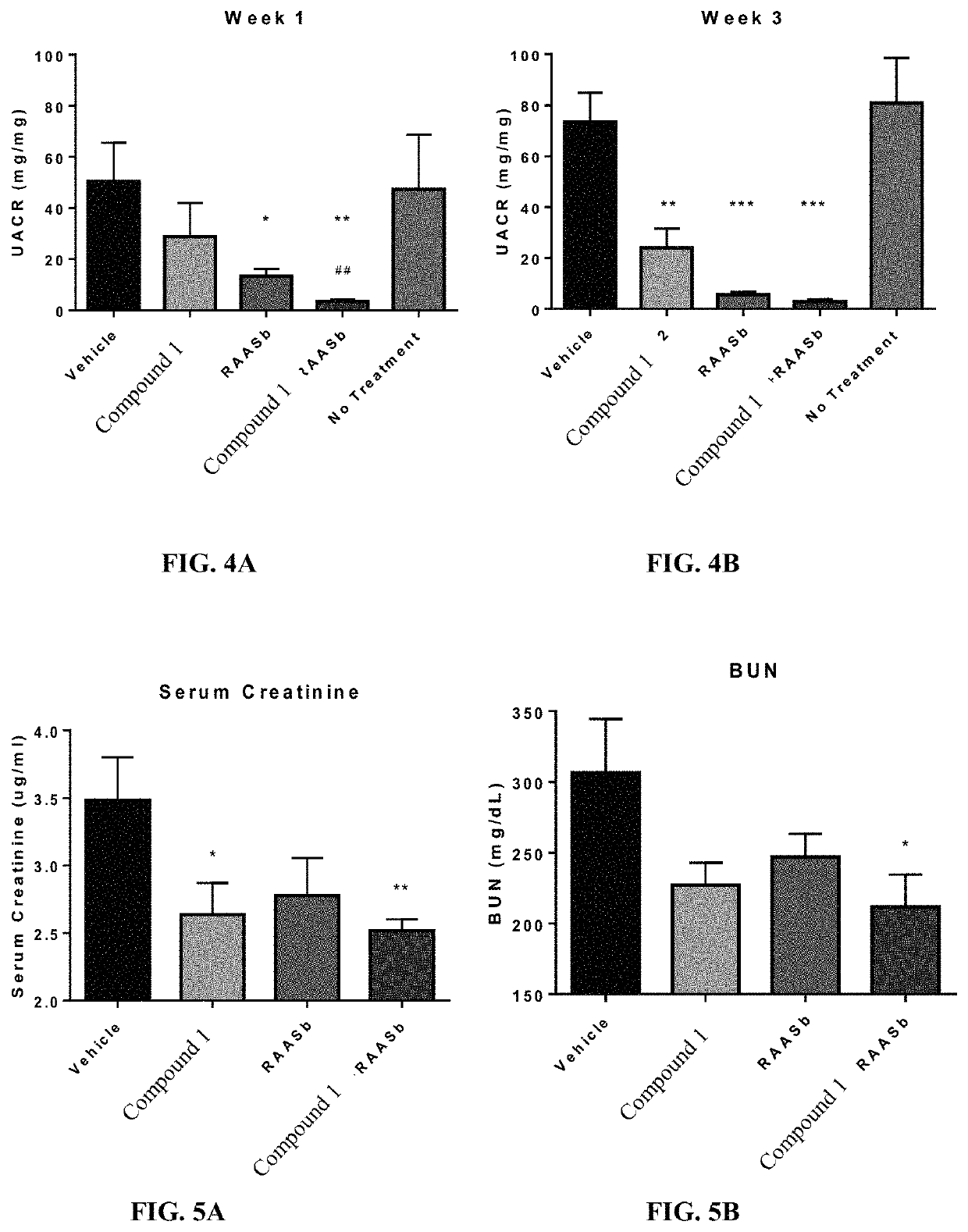
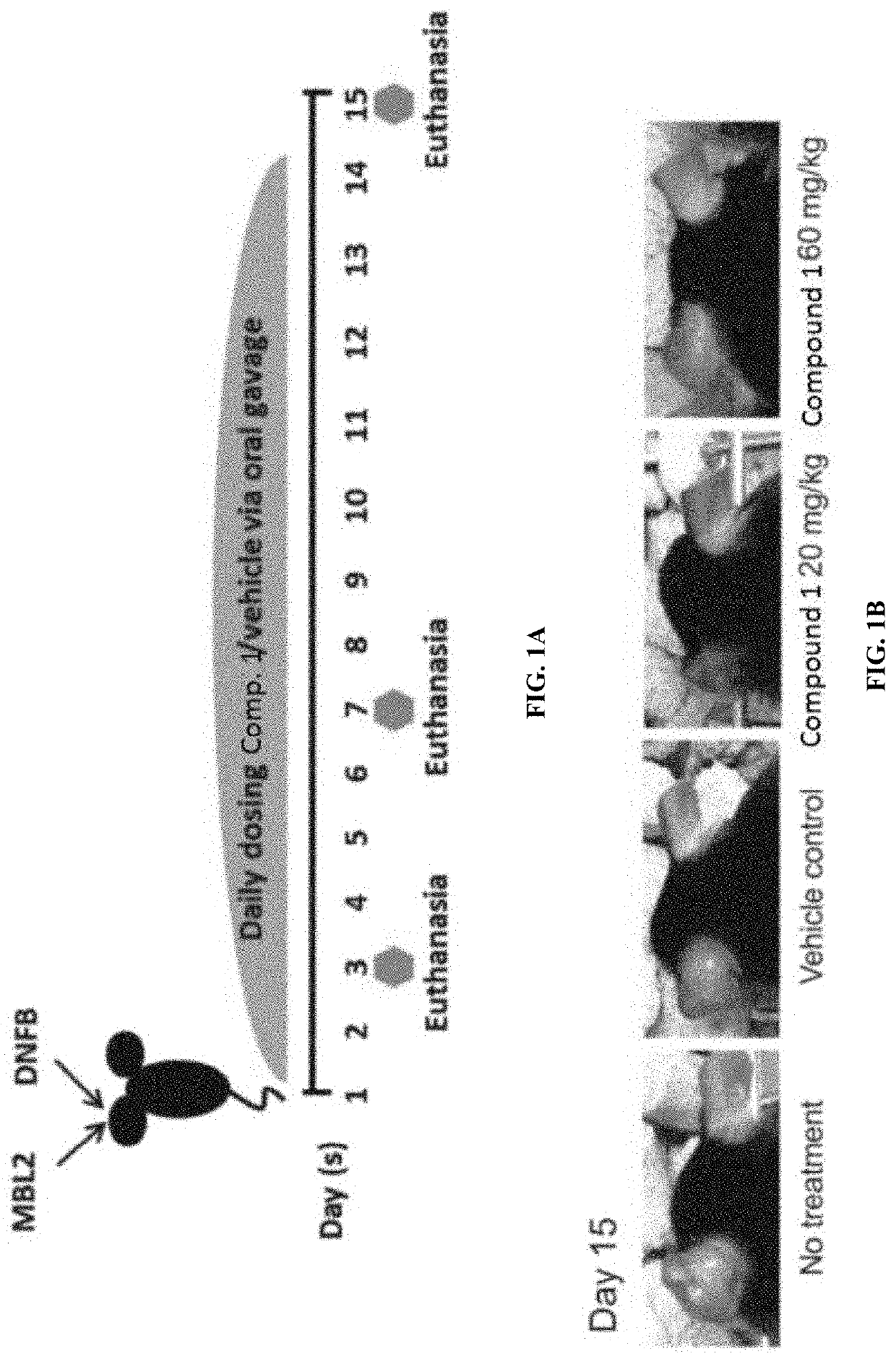
Treatment of focal segmental glomerulosclerosis with CCR2 antagonists
ActiveUS10758540B2Urinary disorderHeterocyclic compound active ingredientsSegmental glomerulosclerosisCcr2 antagonist
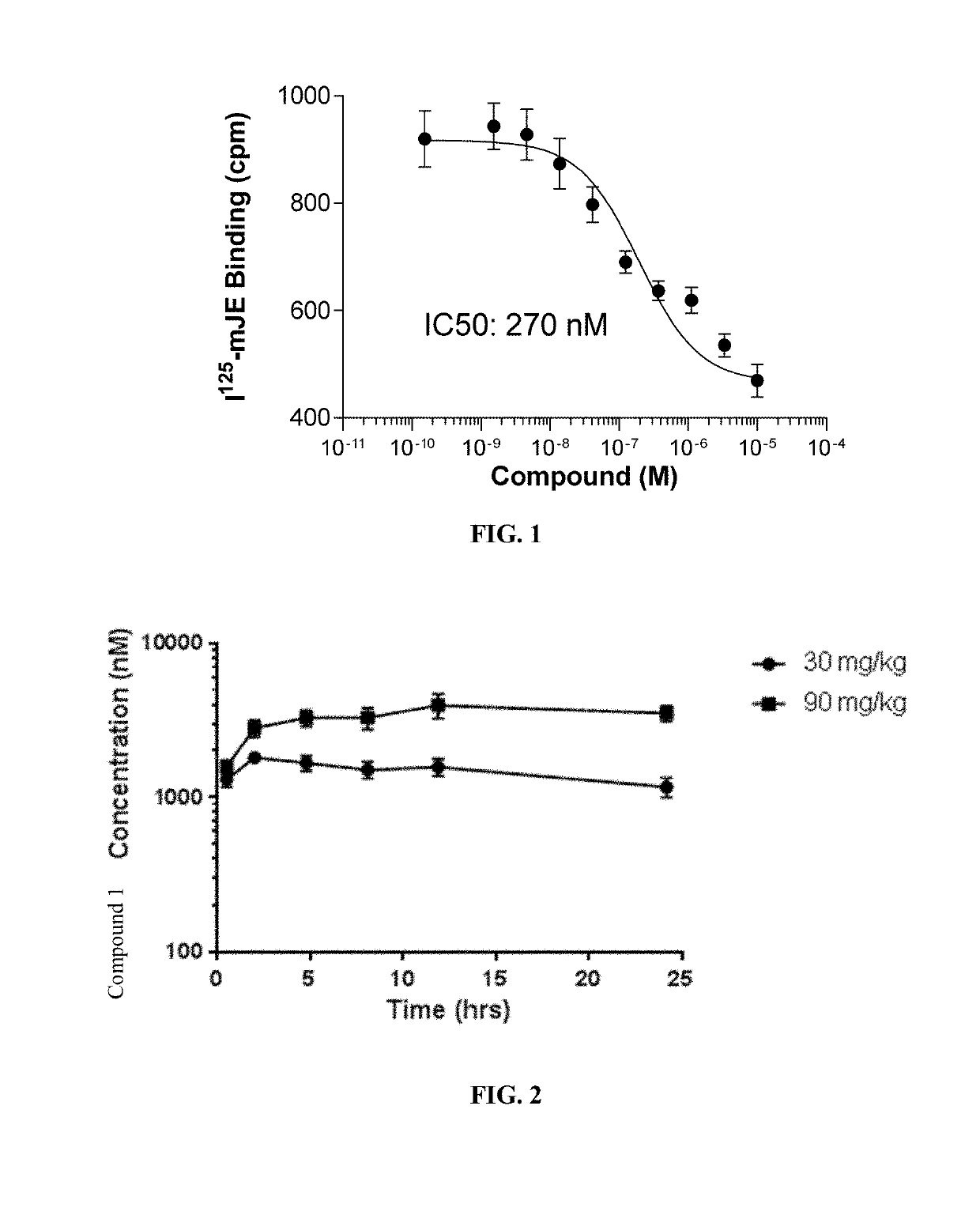
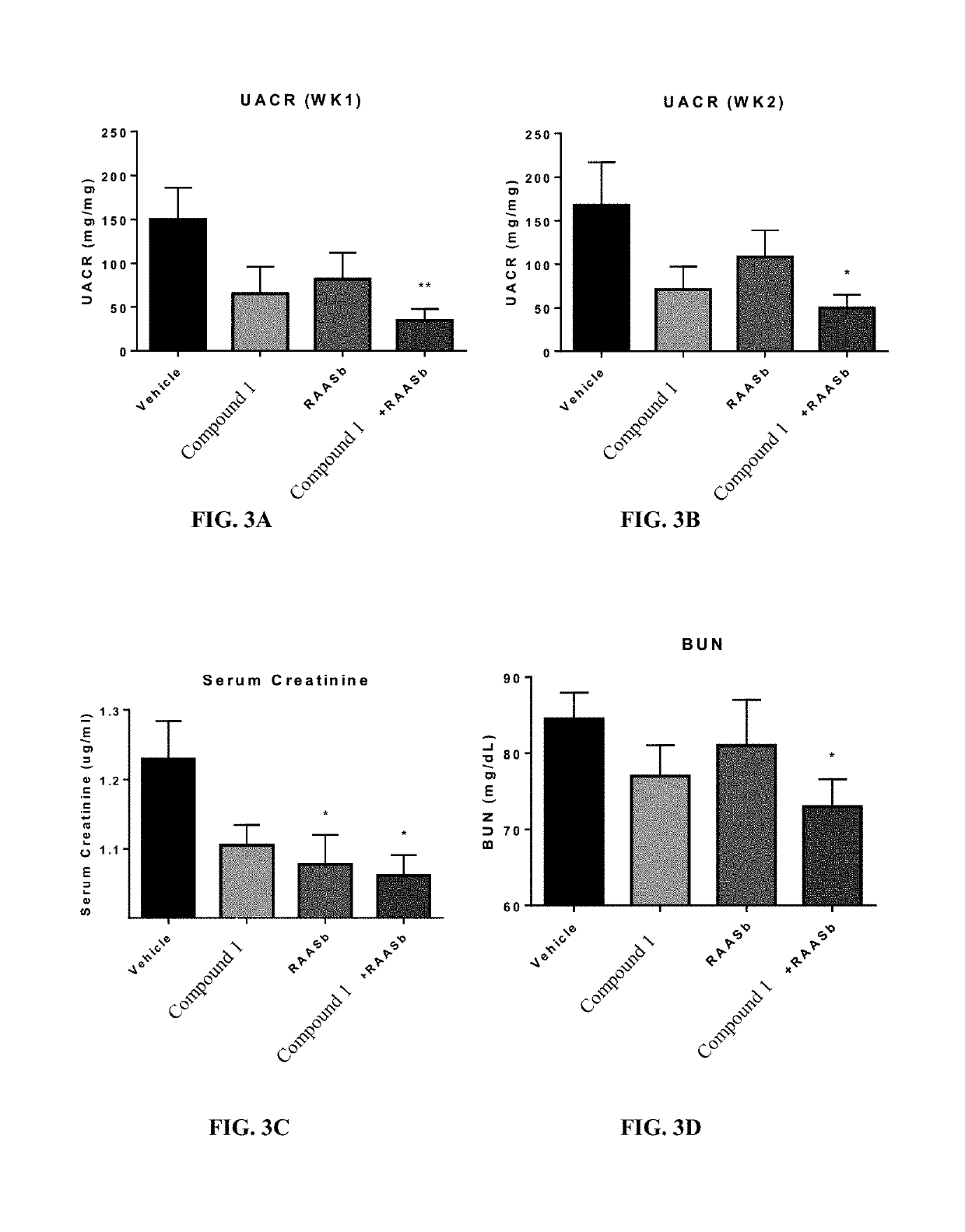
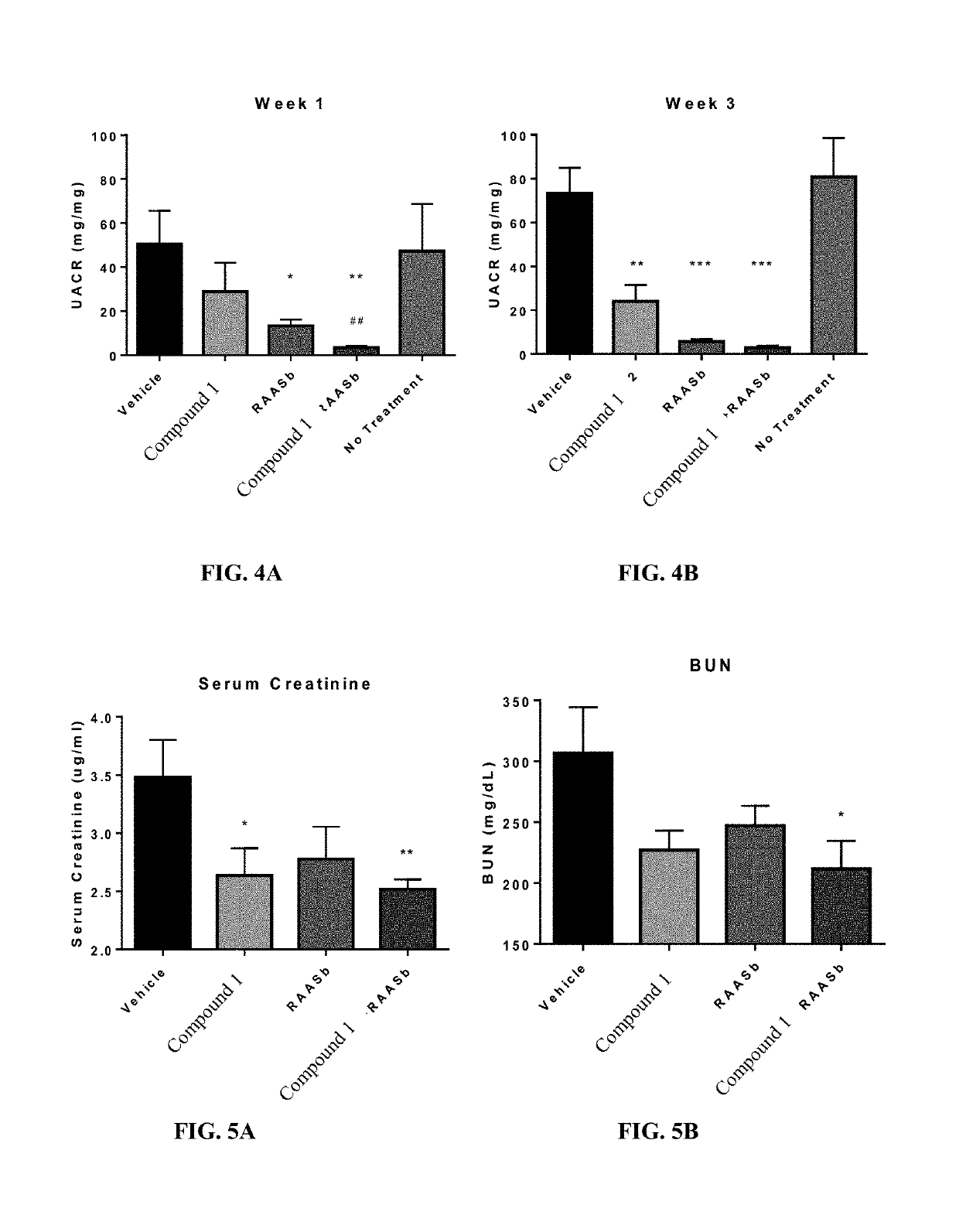
Provided herein are methods of treating focal segmental glomerulosclerosis, said methods include administering to a subject in need thereof a therapeutically effective amount of a CCR2 antagonist. In some embodiments, the CCR2 antagonist is used in monotherapy. In some embodiments, the CCR2 antagonist is used in combination therapy. In some embodiments, the additional therapeutic agent is a RAAS blocker and / or an endothelin receptor inhibitor. The CCR2 antagonist may have the structure of formula (I).
Owner:CHEMOCENTRYX INC
Method of treating liver fibrosis
A method of treating liver fibrosis with CCR2 antagonists is provided. The liver fibrosis may be associated with non-alcoholic steatohepatitis (NASH), non-alcoholic fatty liver disease (NAFLD), emerging cirrhosis, non-cirrhotic hepatic fibrosis, type 2 diabetes mellitus (T2DM) or metabolic syndrome (MS).
Owner:CHEMOCENTRYX INC
Treatment of focal segmental glomerulosclerosis with ccr2 antagonists
ActiveUS20190134042A1Urinary disorderHeterocyclic compound active ingredientsSegmental glomerulosclerosisCcr2 antagonist
Provided herein are methods of treating focal segmental glomerulosclerosis, said methods include administering to a subject in need thereof a therapeutically effective amount of a CCR2 antagonist. In some embodiments, the CCR2 antagonist is used in monotherapy. In some embodiments, the CCR2 antagonist is used in combination therapy. In some embodiments, the additional therapeutic agent is a RAAS blocker and / or an endothelin receptor inhibitor.
Owner:CHEMOCENTRYX INC
Methods of treating solid tumors with ccr2 antagonists
PendingUS20210346361A1Reduce in quantityOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsChemokine Receptor AntagonistCcr2 antagonist
The present disclosure provides, inter alia, methods of treating a solid-tumor by administering an effective amount of a Chemokine Receptor 2 (CCR2) antagonist. Also provided herein are methods of reducing the number of macrophages in a solid tumor microenvironment, said method comprising administering effective amount of a Chemokine Receptor 2 (CCR2) antagonist. In an additional aspect, the current disclosure further provides methods of increasing the number CD8+ T cells in a solid tumor microenvironment, said method comprising administering effective amount of a Chemokine Receptor 2 (CCR2) antagonist. In some embodiments, the CCR2 antagonist has the formula I or Formula III:
Owner:CHEMOCENTRYX INC +1
Methods of treating solid tumors with ccr2 antagonists
ActiveUS20190209573A1Reduce in quantityOrganic active ingredientsAntineoplastic agentsCcr2 antagonistChemokine Receptor Antagonist
The present disclosure provides, inter alia, methods of treating a solid-tumor by administering an effective amount of a Chemokine Receptor 2 (CCR2) antagonist. Also provided herein are methods of reducing the number of macrophages in a solid tumor microenvironment, said method comprising administering effective amount of a Chemokine Receptor 2 (CCR2) antagonist. In an additional aspect, the current disclosure further provides methods of increasing the number CD8+ T cells in a solid tumor microenvironment, said method comprising administering effective amount of a Chemokine Receptor 2 (CCR2) antagonist. In some embodiments, the CCR2 antagonist has the formula I:
Owner:RGT UNIV OF CALIFORNIA +1
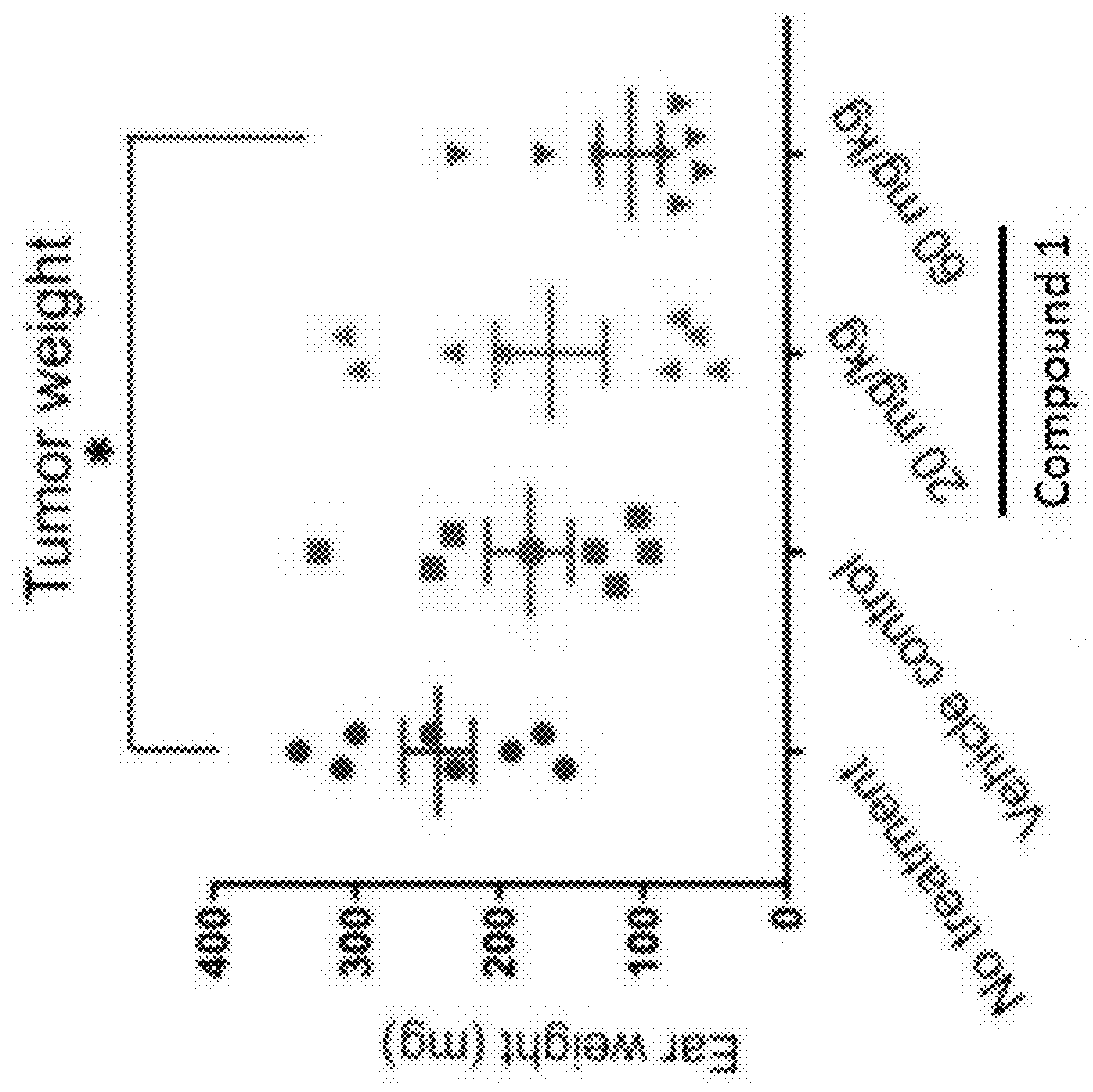
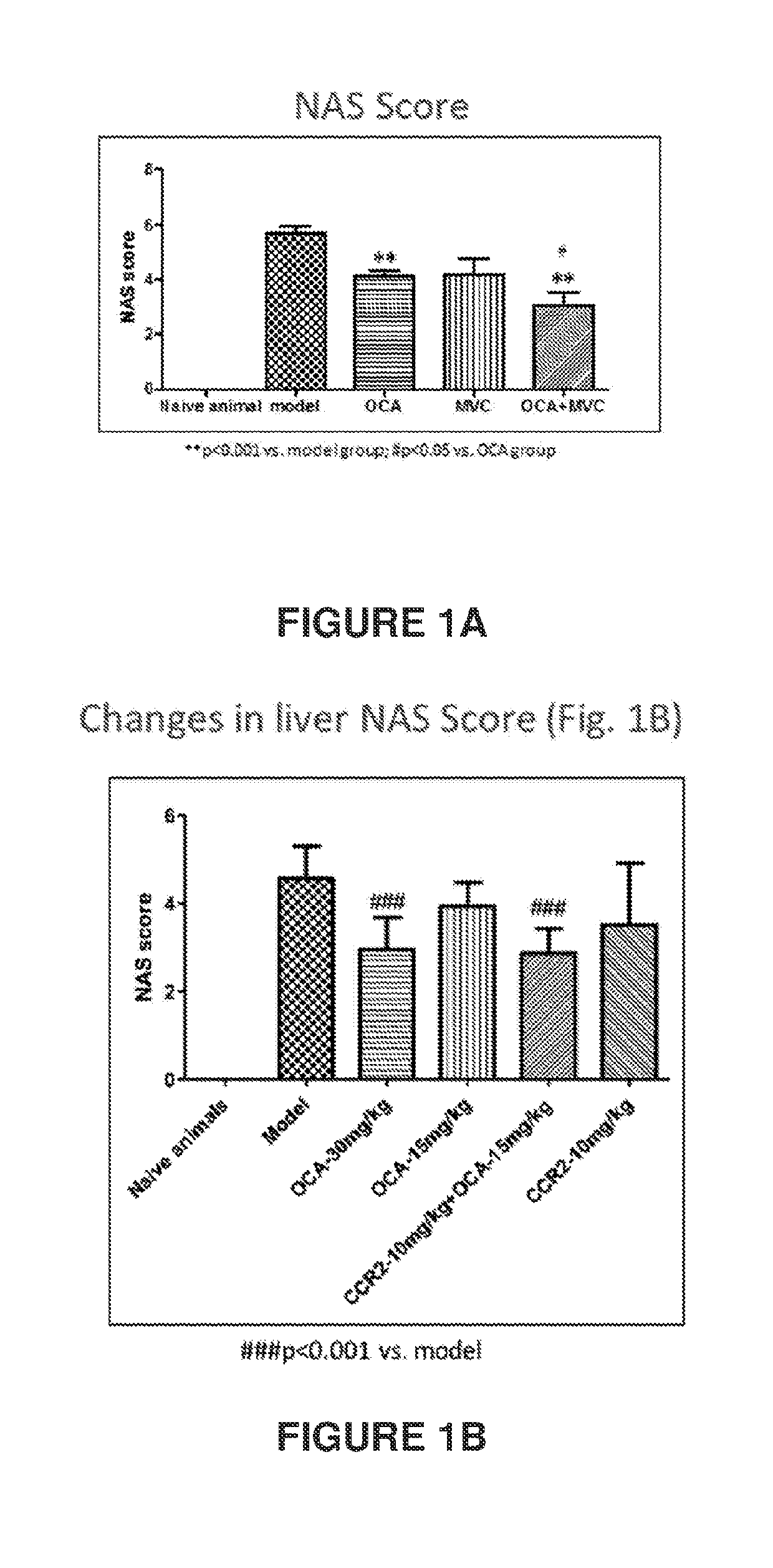
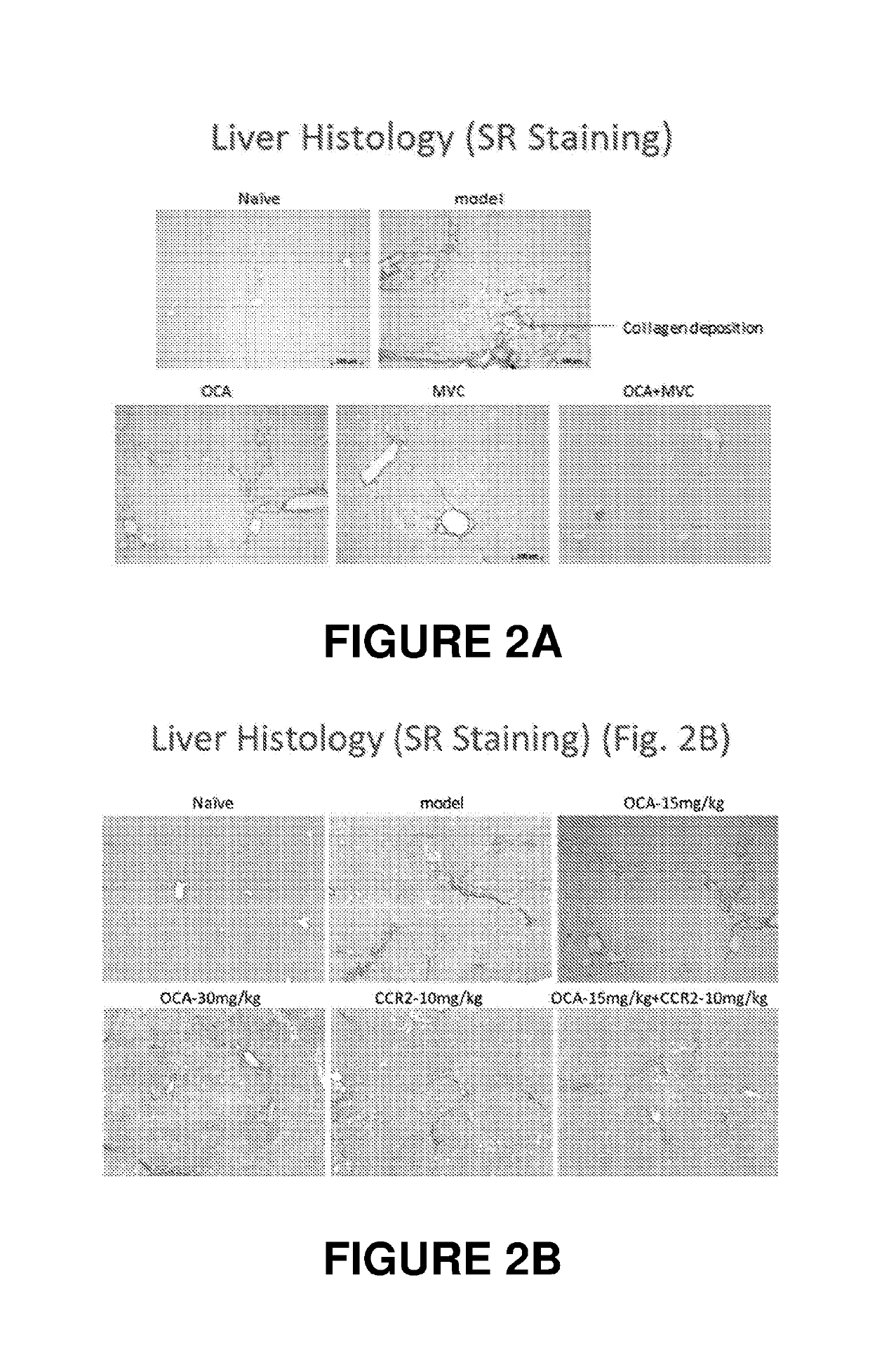
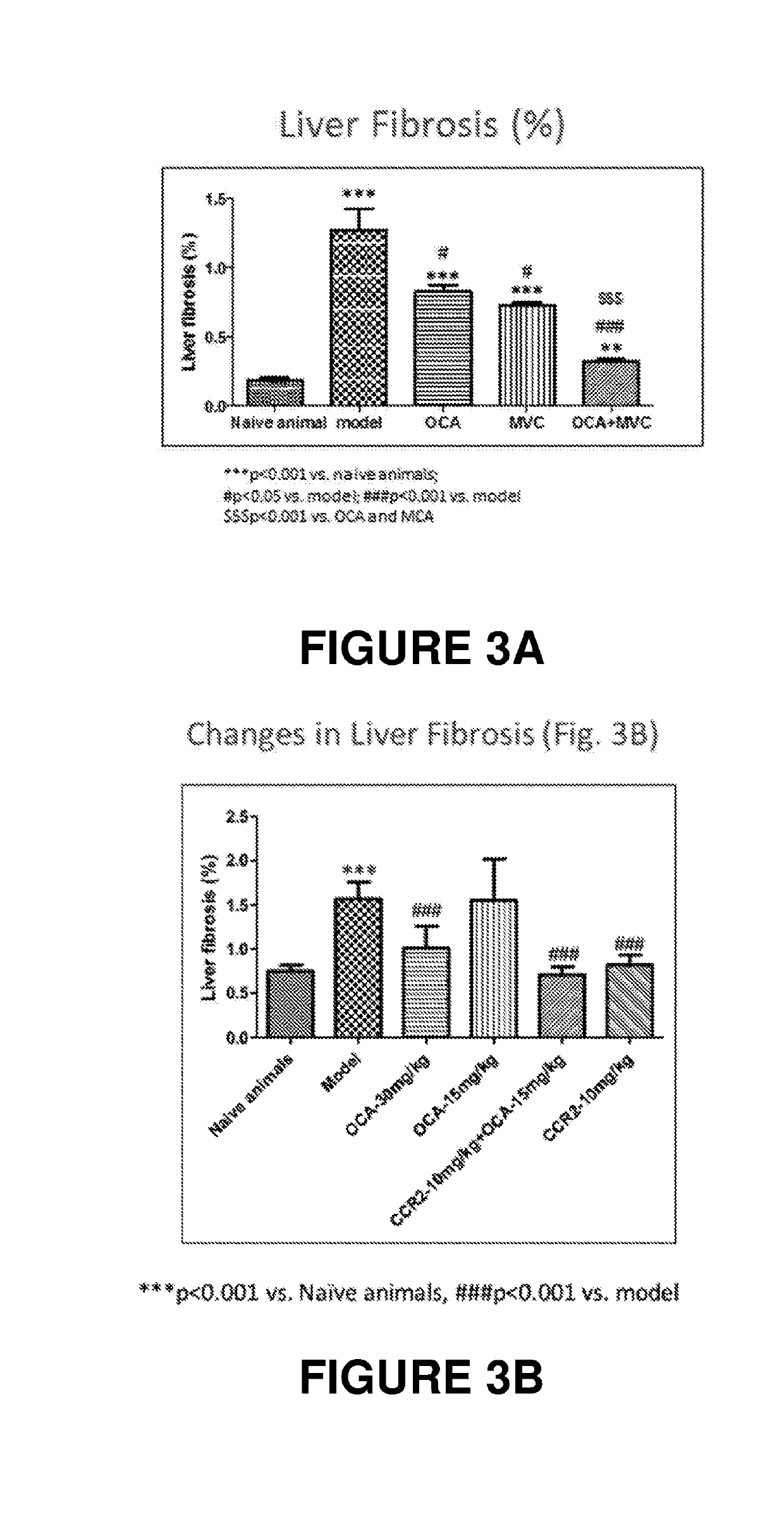
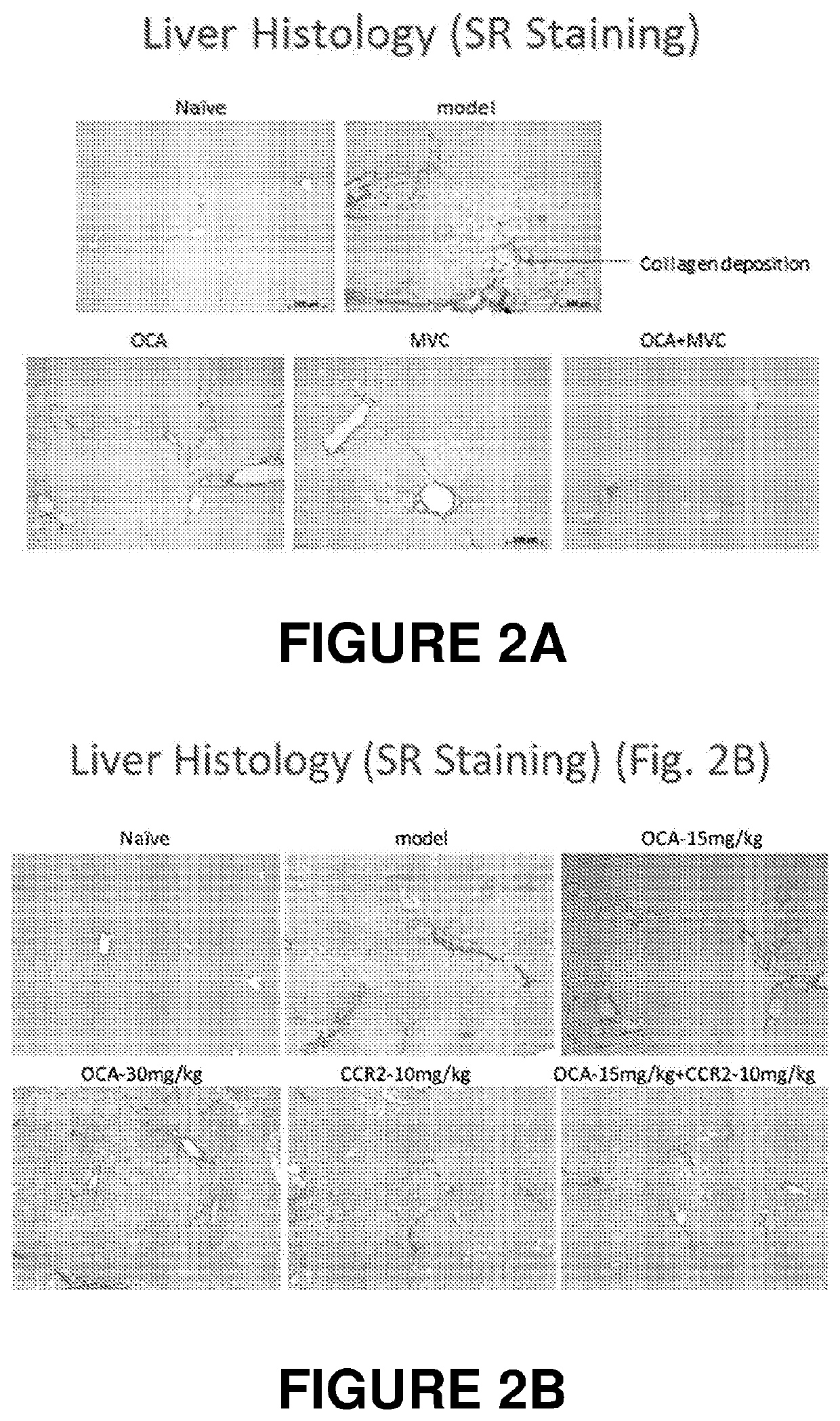
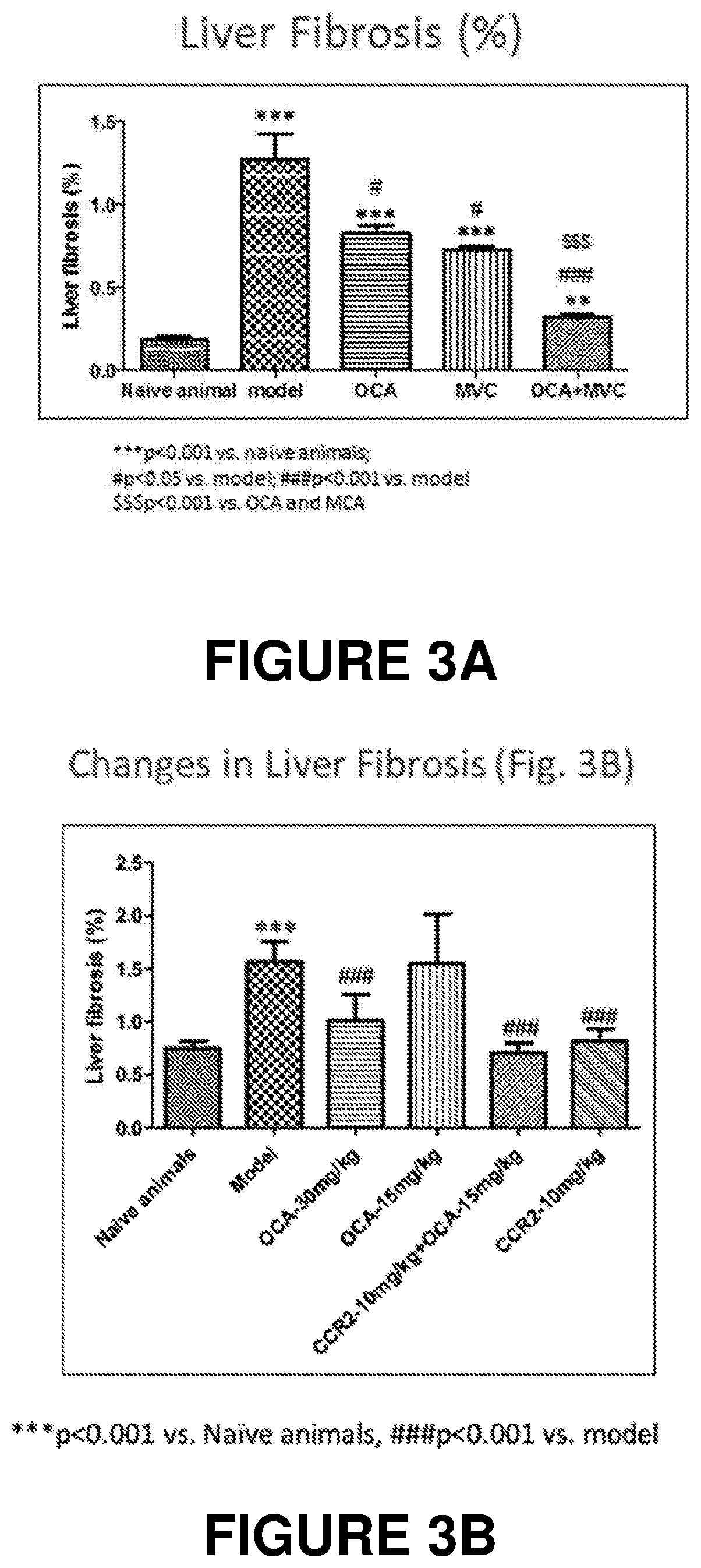
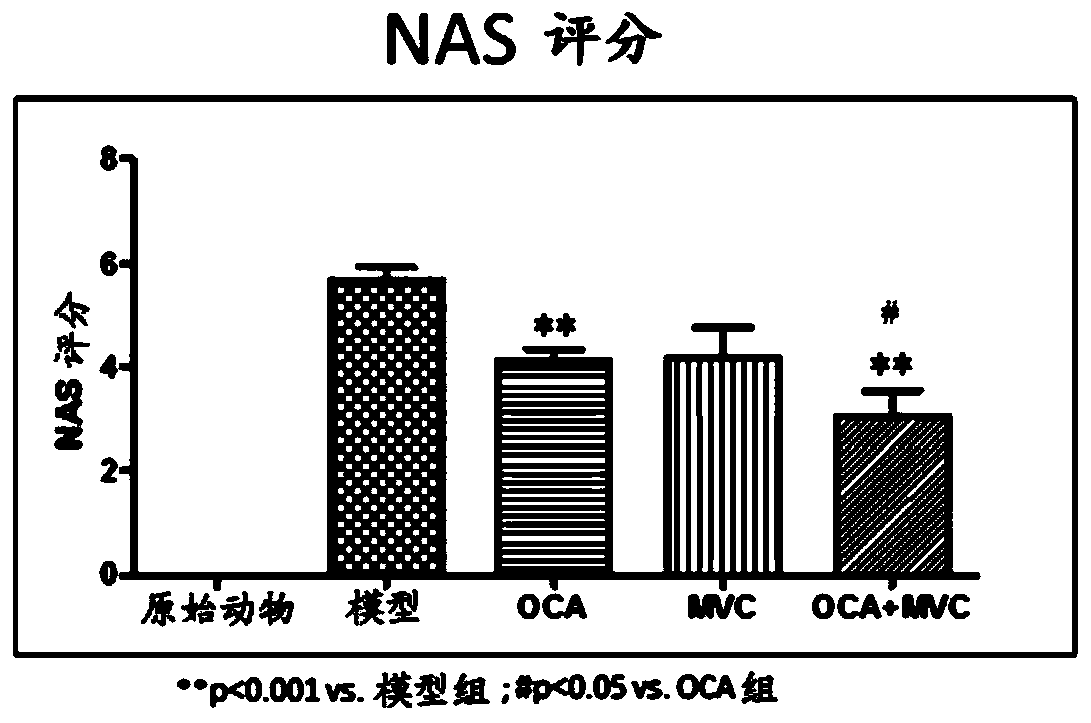
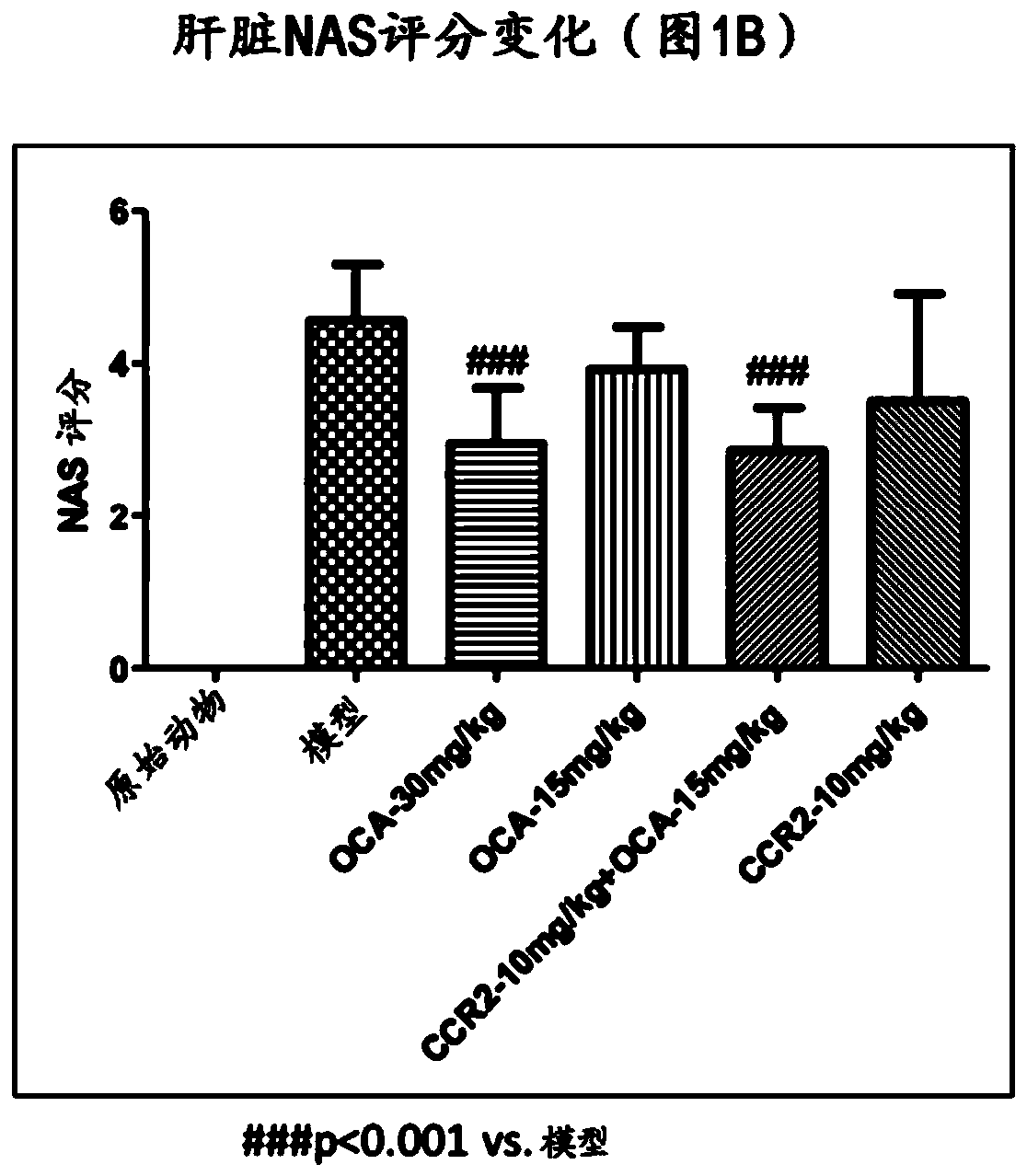
Combination therapy for nonalcoholic steatohepatitis (NASH) and liver fibrosis
ActiveUS20190321377A1Less side effectsTreating NASHDigestive systemUrinary disorderDiseaseVicriviroc
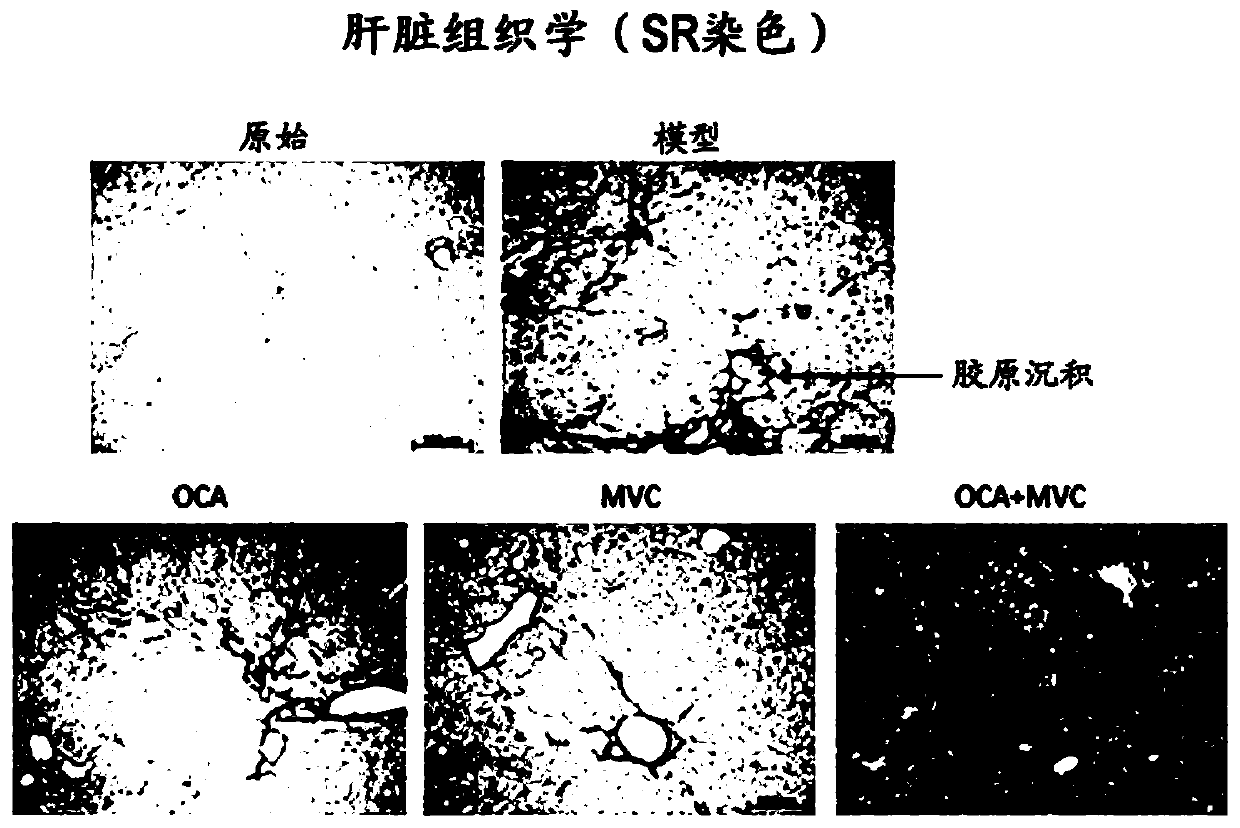
The innovation is directed to a method for treating a CCR5 and / or CCR2 mediated disease such as nonalcoholic steatohepatitis (NASH) comprising administering an effective amount of a C-C Chemokine receptor 5 (CCR5) antagonist (e.g., maraviroc or vicriviroc or cenicriviroc) and / or an effective amount of a C-C Chemokine receptor 2 (CCR2) antagonist, or a CCR5 / CCR2 antagonist together with an effective amount of farnesoid X receptor (FXR) agonist (e.g., obeticholic acid (OCA)). An “effective amount” can be a regular clinical dose of either agent alone or a reduced dose of the FXR receptor agonist and / or the CCR5 / CCR2 antagonist. The combination is effective to treat NASH with (1) enhanced efficacy and (2) substantial reduction of side effects, particularly those associated with administration of OCA or its analogues, namely less effect on liver enzyme elevation, and less severity and frequency of pruritus (3). The fixed dose combination provides for better efficacy and safety profile.
Owner:MODUNEX BIO CORP
Methods of treating solid tumors with CCR2 antagonists
ActiveUS11154556B2Reduce in quantityOrganic active ingredientsAntineoplastic agentsChemokine Receptor AntagonistCcr2 antagonist
The present disclosure provides, inter alia, methods of treating a solid-tumor by administering an effective amount of a Chemokine Receptor 2 (CCR2) antagonist. Also provided herein are methods of reducing the number of macrophages in a solid tumor microenvironment, said method comprising administering effective amount of a Chemokine Receptor 2 (CCR2) antagonist. In an additional aspect, the current disclosure further provides methods of increasing the number CD8+ T cells in a solid tumor microenvironment, said method comprising administering effective amount of a Chemokine Receptor 2 (CCR2) antagonist. In some embodiments, the CCR2 antagonist has the formula I:
Owner:RGT UNIV OF CALIFORNIA +1
Combination therapy for nonalcoholic steatohepatitis (NASH) and liver fibrosis
ActiveUS11160813B2Less side effectsEffective treatmentDigestive systemUrinary disorderVicrivirocDisease
Owner:MODUNEX BIO CORP
Combination therapy for nonalcoholic steatohepatitis (NASH) and liver fibrosis
InactiveCN110352059AImprove securityFull dose reductionDigestive systemUrinary disorderVicrivirocDisease
The innovation is directed to a method for treating a CCR5 and / or CCR2 mediated disease such as nonalcoholic steatohepatitis (NASH) comprising administering an effective amount of a C-C Chemokine receptor 5 (CCR5) antagonist (e.g., maraviroc or vicriviroc or cenicriviroc) and / or an effective amount of a C-C Chemokine receptor 2 (GGR2) antagonist, or a CCR5 / CCR2 antagonist together with an effective amount of farnesoid X receptor (FXR) agonist (e.g., obeticholic acid (OCA)). An effective amount can be a regular clinical dose of either agent alone or a reduced dose of the FXR receptor agonist and / or the CCR5 / CCR2 antagonist. The combination is effective to treat NASH with (1) enhanced efficacy and (2) substantial reduction of side effects, particularly those associated with administration ofOCA or its analogues, namely less effect on liver enzyme elevation, and less severity and frequency of pruritus (3). The fixed dose combination provides for better efficacy and safety profile.
Owner:CHAOYI BIOPHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com