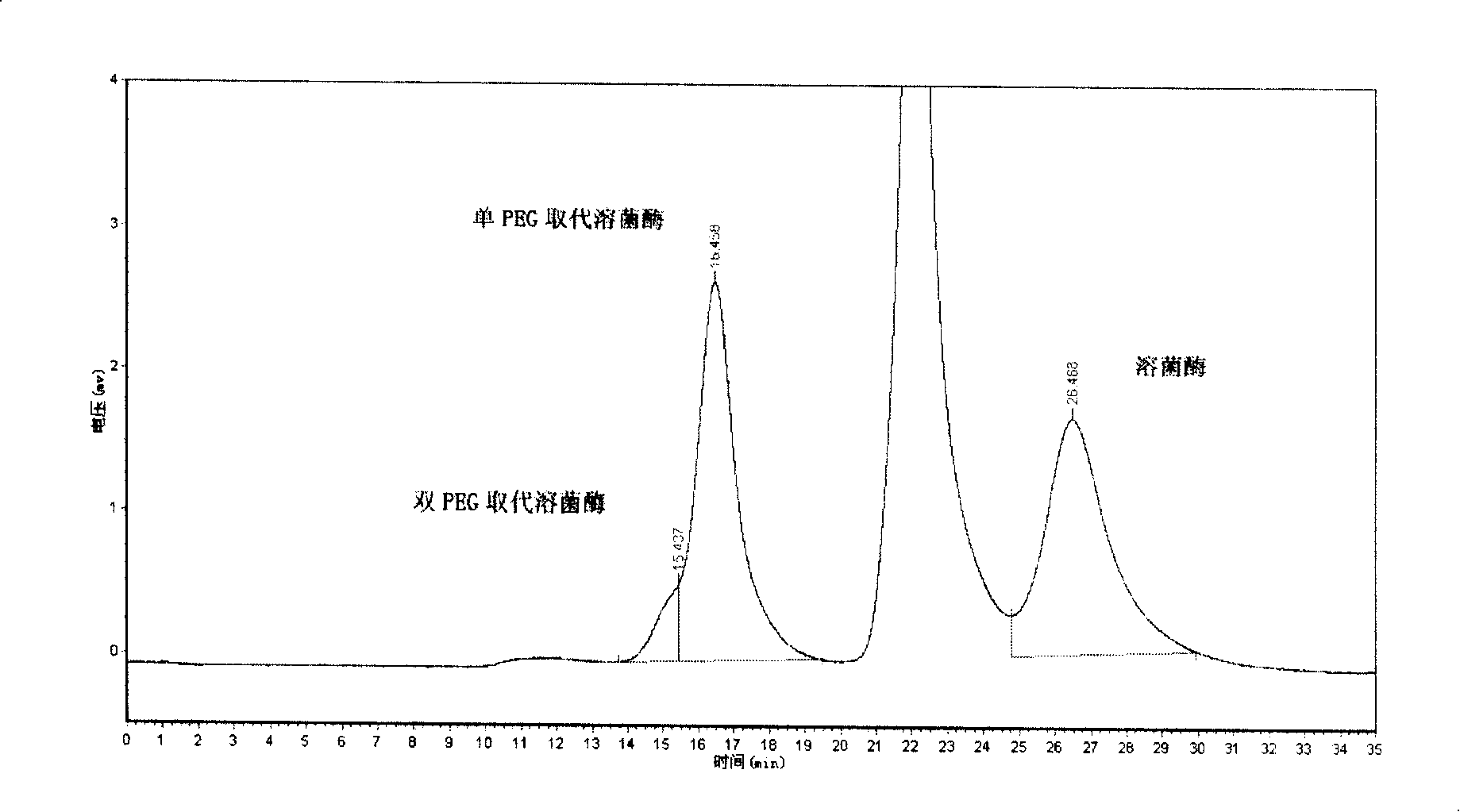
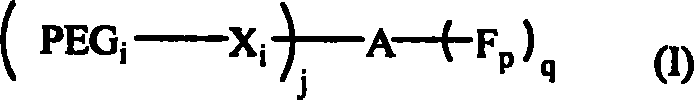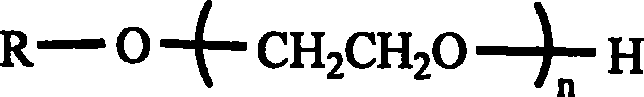Polyglycol active derivative with oligopeptide as framework, preparation method thereof and conjugate of the same and pharmaceutical molecule
A technology of polyethylene glycol and drug molecules, which is applied in the fields of genetic material components, chemical instruments and methods, and medical preparations of non-active ingredients, etc., can solve the problems of poor binding stability and low degree of modification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Synthesis of active derivatives of double-chain polyethylene glycol formed by polyethylene glycol acetic acid and Gly-Lys dipeptide (1)
[0081]
[0082] Dissolve 20 g of polyethylene glycol monomethyl ether acetate-N-hydroxysuccinimide ester (mPEG-SCM) with a molecular weight of 20,000 in phosphate buffer containing 1 g of Gly-Lys dipeptide, pH=8.5 , stirred at room temperature for three hours. Adjust the pH to 3 with hydrochloric acid, extract three times with dichloromethane, combine the organic phases, dry the organic phases over anhydrous sodium sulfate, evaporate the solvent in vacuo, and add 200 ml of isopropanol to precipitate. The precipitate was filtered and dried in vacuo. Branched PEG acidic products can be further purified by ion-exchange chromatography. Yield: 8 g (40%). NMR (DMSO) 3.50 (br m, hydrogens in PEG), 3.24 (s, 3 hydrogens), 4.15 (t, 2 hydrogens).
[0083] 0.5 g of branched PEG acid (prepared from the previous step) was dissolved in 5 mL o...
Embodiment 2
[0085] Synthesis of active derivatives of double-chain polyethylene glycol formed by polyethylene glycol and Gly-Lys dipeptide (2)
[0086]
[0087] Dissolve 20 g of polyethylene glycol monomethyl ether orthocarbonate-N-hydroxysuccinimide ester (mPEG-SC) with a molecular weight of 20,000 in borate buffer containing 1 g of Gly-Lys dipeptide, pH =10.2, stirred overnight at room temperature. Adjust the pH to 3 with hydrochloric acid, extract three times with dichloromethane, combine the organic phases, dry the organic phases over anhydrous sodium sulfate, evaporate the solvent in vacuo, and add 200 ml of isopropanol to precipitate. The precipitate was filtered and dried in vacuo. Branched PEG acidic products can be further purified by ion-exchange chromatography. Yield: 8 g (40%). NMR (DMSO) 3.50 (br m, hydrogens in PEG), 3.24 (s, 3 hydrogens), 4.32 (t, 2 hydrogens).
[0088]0.5 g of branched PEG acid (prepared from the previous step) was dissolved in 5 mL of dichlorometha...
Embodiment 3
[0090] Synthesis of active derivatives of double-chain polyethylene glycol formed by polyethylene glycol and Gly-Lys (Gly) tripeptide (3)
[0091]
[0092] Dissolve 20 g of polyethylene glycol monomethyl ether orthocarbonate-N-hydroxysuccinimide ester (mPEG-SC) with a molecular weight of 20,000 in borate buffer containing 1.2 g of Gly-Lys (Gly) polypeptide , pH=10.2, stirred overnight at room temperature. Adjust the pH to 3 with hydrochloric acid, extract three times with dichloromethane, combine the organic phases, dry the organic phases over anhydrous sodium sulfate, evaporate the solvent in vacuo, and add 200 ml of isopropanol to precipitate. The precipitate was filtered and dried in vacuo. Branched PEG acidic products can be further purified by ion-exchange chromatography. Yield: 8 g (40%). NMR (DMSO) 3.50 (br m, hydrogens in PEG), 3.24 (s, 3 hydrogens), 4.32 (t, 2 hydrogens).
[0093] 0.5 g of branched mPEG acid (prepared from the previous step) was dissolved in 5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap