Crystalline sugar compositions and method of making
A technology of crystal and furanose, applied in the field of crystallization of crystal pivaloyl furanose and pivaloyl furanose, can solve problems such as the inability to easily expand the scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
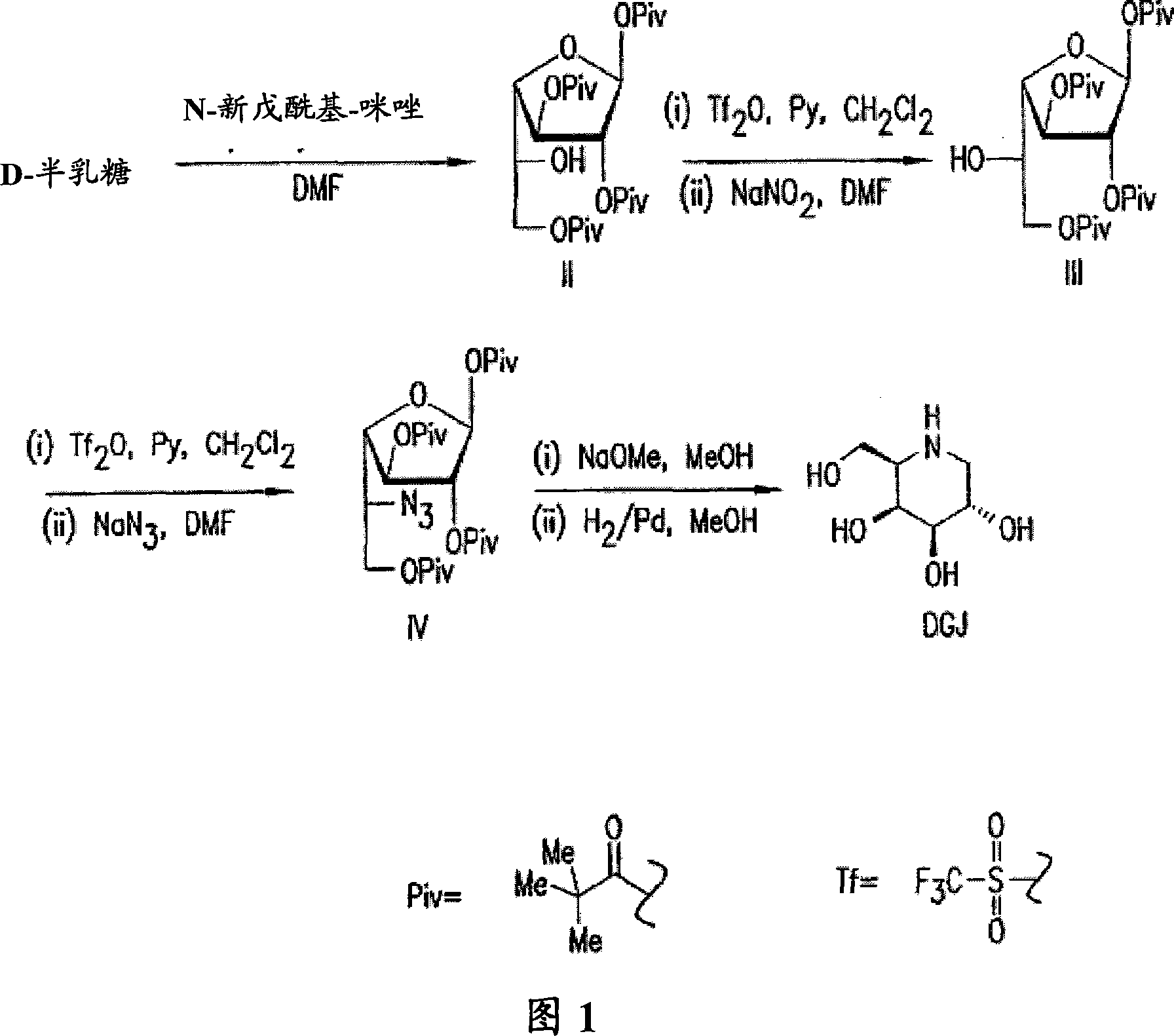
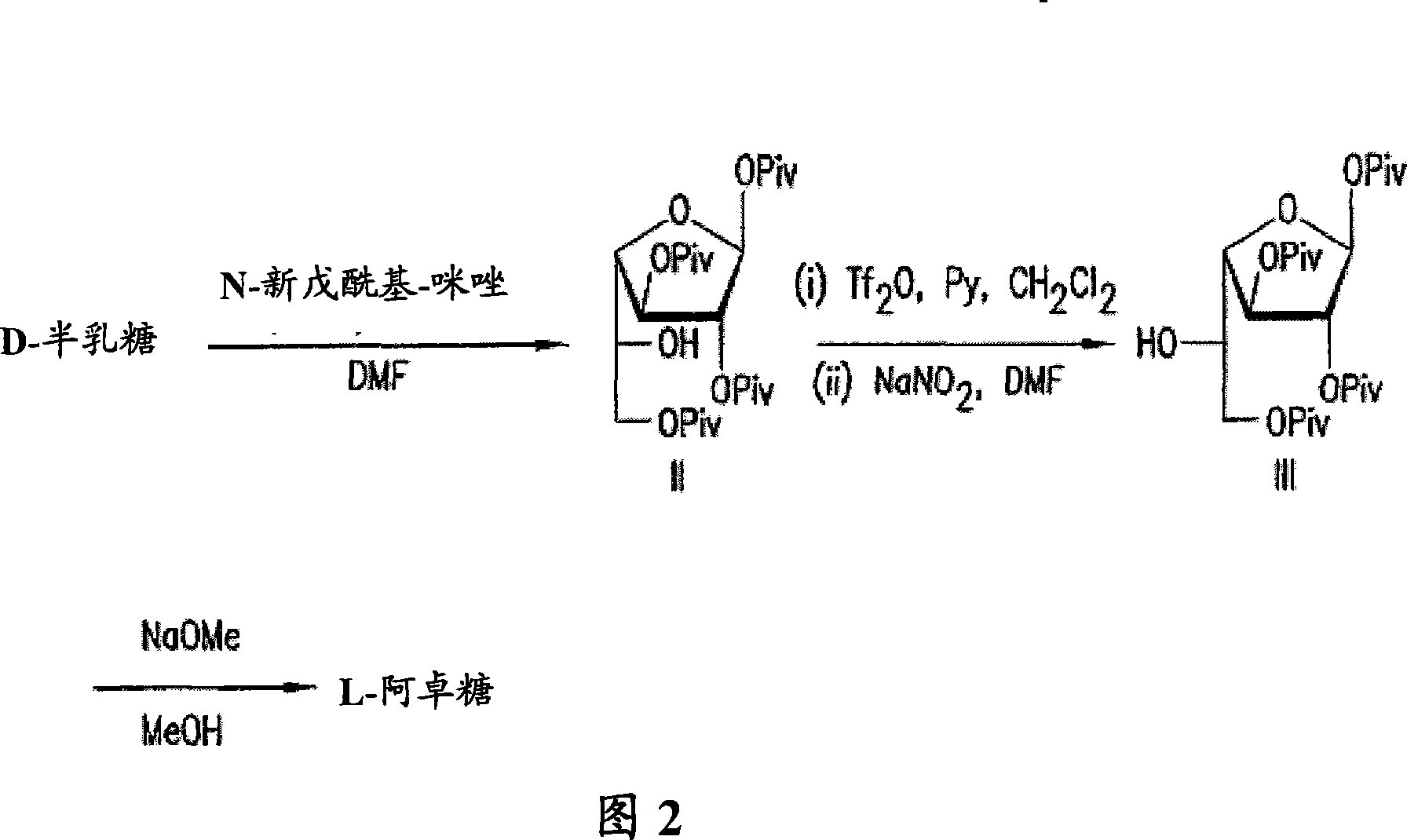
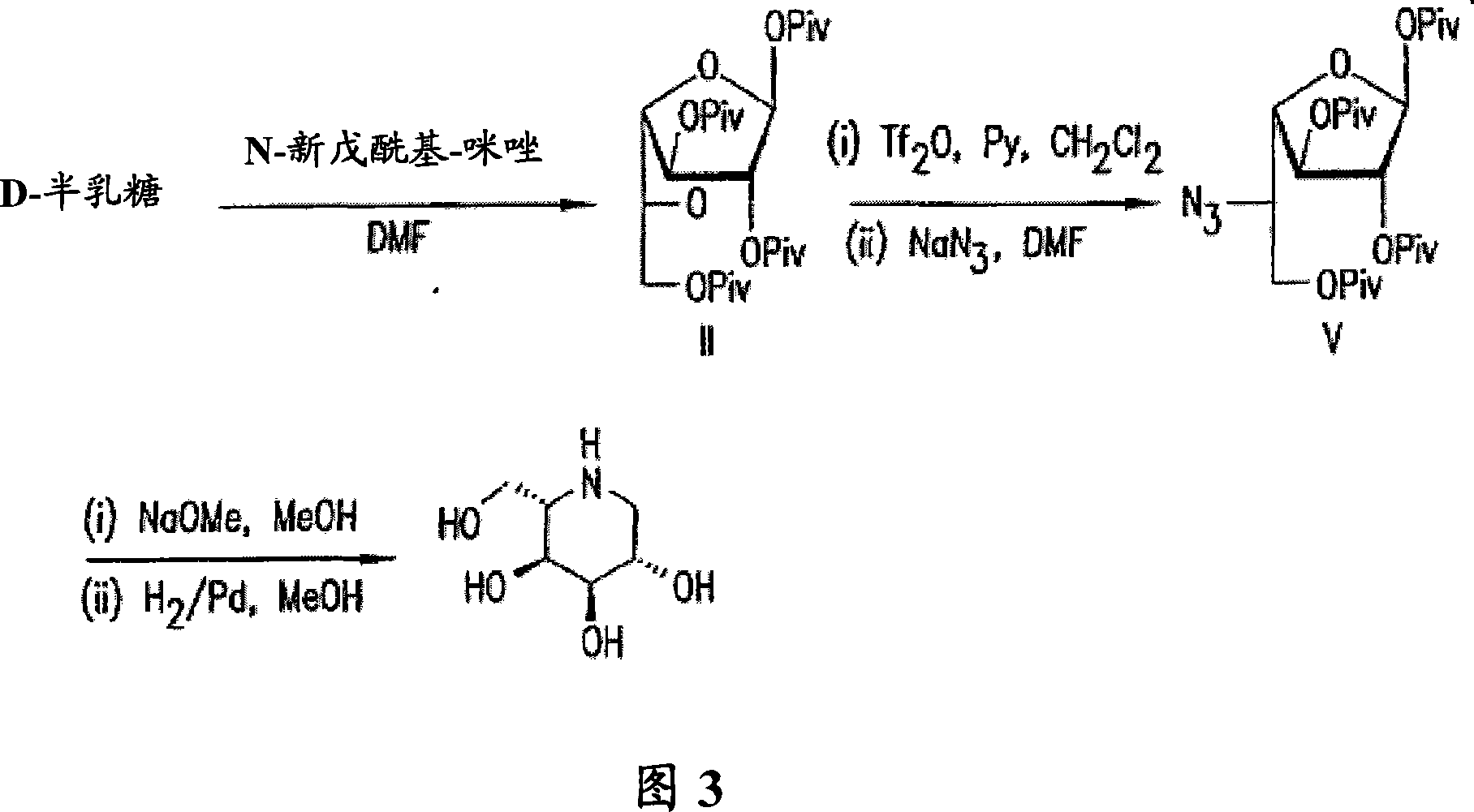
[0089] Example 1: Synthesis and identification of crystal 1,2,3,6-tetrapivaloyl-α-D-galactofuranose (II) 1-(trimethylacetyl)imidazole (pivaloyl imidazole) ( 42.2kg, 5-fold excess) was dissolved in DMF (90kg) and heptane (3.4kg), and the solution was warmed to 60°C. D-galactose (10 kg) was added to the solution, and the mixture was heated to 75°C. The reaction is exothermic to a temperature of 90-100°C. After the exotherm subsides, the reaction is maintained at 80-100°C until the reaction is complete. The progress of the reaction was monitored by TLC (hexane:ethyl acetate=4:1). To visualize progress, TLC was subsequently stained with dilute sulfuric acid and heated; f= 0.5) the reaction was considered complete when the product spot on it became the major component. Immediately after the reaction was complete, the reaction product was transferred to a mixture containing water (200 kg) and ice (82 kg). The crude product is isolated from this mixture by crystallization; this c...
Embodiment 2
[0090] Example 2: Preparation and identification of crystalline 1,2,3,6-tetrapivaloyl-α-L-altrofuranose (III)
[0091] A solution of pyridine (3.82 kg) in dichloromethane (15 L) was cooled to 0°C under a nitrogen atmosphere. Add trifluoromethanesulfonic anhydride (3.28kg) dropwise at 0°C, followed by dropwise addition of dichloromethane (10L) with 1,2,3,6-tetrapivaloyl-α-D-lactofuranoside (5kg) solution. The reaction mixture was stirred at 0°C for 2 hours, and the completion of the reaction was monitored by TLC (hexane:ethyl acetate=4:1). If the reaction was not complete by this point, an additional portion of trifluoromethanesulfonic anhydride (0.1 kg) was added. At this stage in the reaction, the triflated compound 5-trifluoromethanesulfonyloxy-5-deoxy-1,2,3,6-tetrapivaloyl is formed from galactofuranosides -α-D-Galactofuranoside. The reaction mixture was then washed with cold 6% hydrochloric acid (3 times, 30 L), brine (30 L) and 7.5% sodium bicarbonate solution (30 L)....
Embodiment 3
[0093] Example 3: Preparation and identification of crystalline 5-azido-5-deoxy-1,2,3,6-tetrapivaloyl-α-D-galactofuranose (IV)
[0094] According to the method described in Example 2, the trifluoromethanesulfonated compound, namely 5-trifluoromethanesulfonyloxy-5-deoxy- 1,2,3,6-tetrapivaloyl-α-L-altrofuranose. This compound was reacted with sodium azide (1.6 kg) in DMF (9.5 L). The reaction was carried out using the optimum conditions observed during the transformation reaction. The crude product was crystallized twice from methanol (1.3-1.7 mL / g). On a 5 kg scale, the yield of 5-azido-5-deoxy-1,2,3,6-tetrapivaloyl-α-D-galactofuranose (IV) from III is typically 65-70% (~3.3kg). The product is a white crystalline solid. M.P.103-104℃.IR(KBr, cm -1 ): 2090 (azido, s), 1740 (ester group of pivalate, vs), 1480 (weak), 1280 (C-O, s), 1160 (C-O, vs), 1042 (C-O, weak); 1 HNMR (CDCl 3 , 400 MHz, TMS): δ=1.19(s, 3H), 1.20(s, 3H), 1.22(s, 3H), 1.25(s, 3H), 3.83-3.79(m, 1H), 4.05(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap