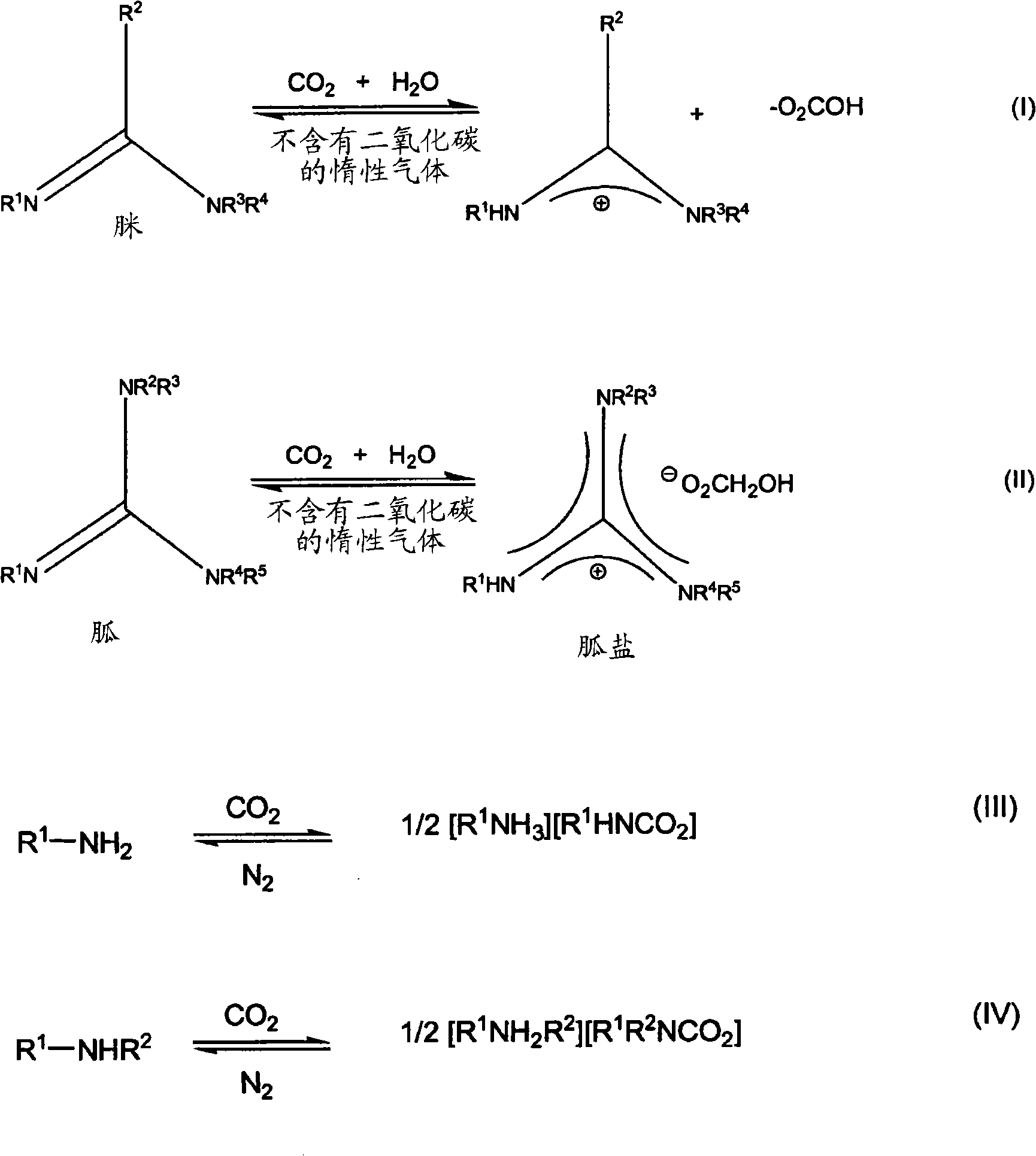
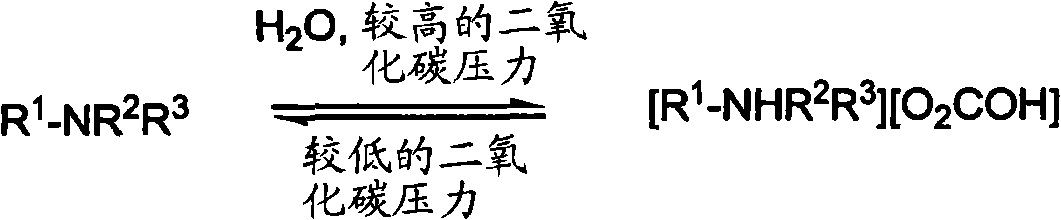
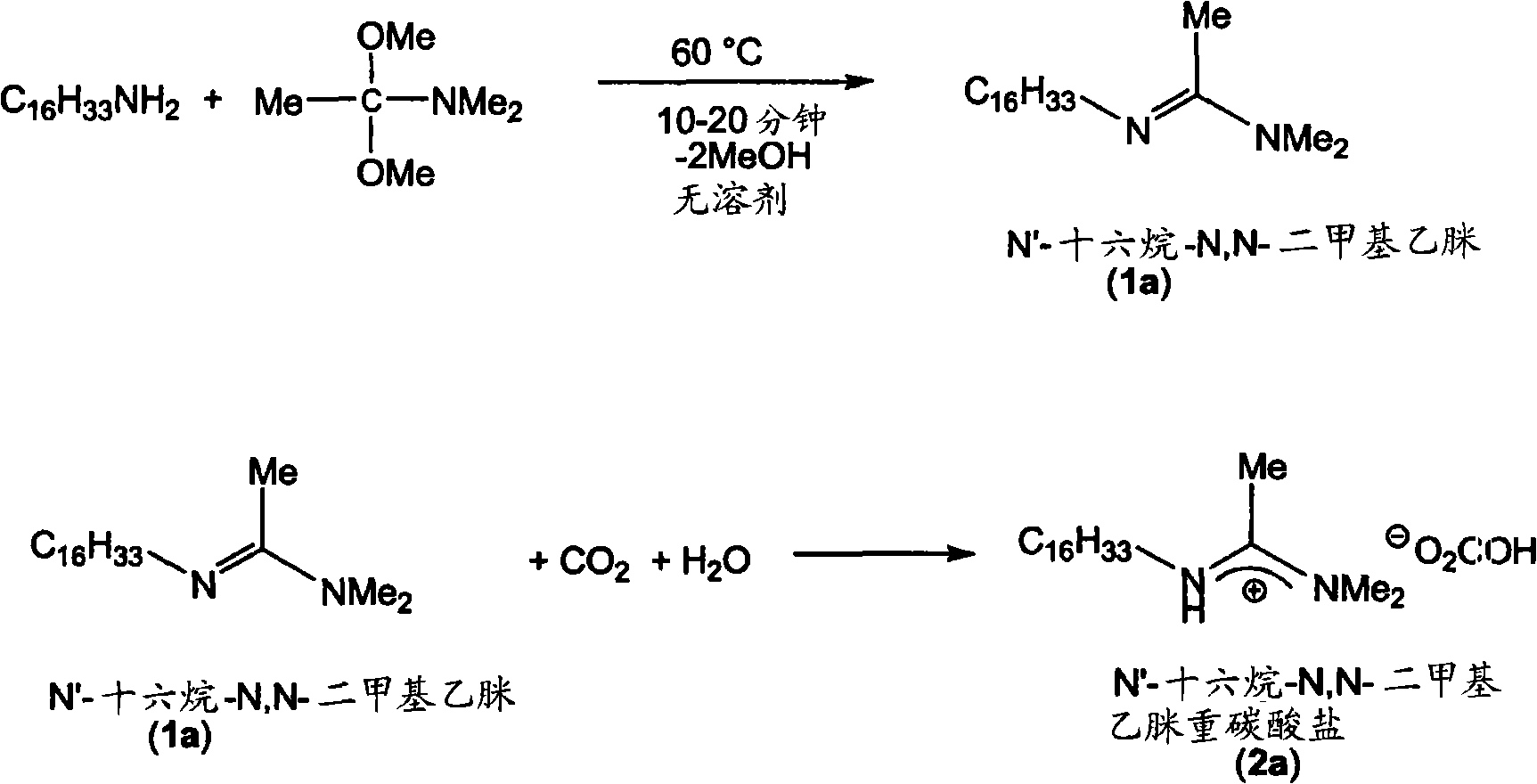
Reversibly switchable surfactants and methods of use thereof
A surfactant and non-surfactant technology, applied in the field of surfactants, can solve the problem of slow photochemical reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 8
[0087] Example 8 describes the determination of the critical micelle concentration of an amidine according to the invention. The charge of the ionic surfactant molecule is such that the ionic surfactant molecule imparts a certain conductivity to its solution. At the critical micelle concentration (CMC), surfactant molecules begin to aggregate into colloidal molecular clusters whose mobility is very different from that of single ions; therefore, plotting conductance against surfactant concentration Rate curve, there will be a titration jump (Patist, A., Hand Book of Applied Surface and Colloid Chemistry, Vol.2 (Ed.: K.Holmberg) of specific conductivity at the critical micelle concentration (CMC) place, John Wiley & Sons, New York, 2002; Schultz, P.C.; Clausse, D.; J. Chem. Ed. 2003, 80, 1053). Critical micelle concentration (CMC) is an indicator of surfactant efficiency (D.Myers, Surfactant Science and Technology, 3 rd ed., John Wiley & Sons Inc.: New York, U.S.A., 2006). A...
Embodiment 4
[0097] Example 4 shows that ammonium bicarbonate 2a can be decomposed between about 50°C and about 63°C and releases carbon dioxide and water. Carbon dioxide loss between room temperature and 100°C is desirable; therefore measured temperatures between 50°C and 63°C are within the desired range. If the decomposition temperature of 2a is higher than 100° C., the compound of the invention is unlikely to release carbon dioxide even in boiling water; ammonium bicarbonate is too stable to be converted. If the decomposition temperature is lower than room temperature, ammonium bicarbonate is unstable at room temperature, and it will switch to the closed state to give a neutral amidine.
[0098] If isolation of the switchable switchable surfactant of the present invention is desired, it can be isolated in any form by reverse solubility. When the switched-on form (salt form) is switched off, the switchable surfactant separates from the aqueous solution, allowing it to be easily recover...
Embodiment 1
[0118] Embodiment 1.N '-alkyl-N, N-dimethyl acetamidine compound Synthetic and Qualitative
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap