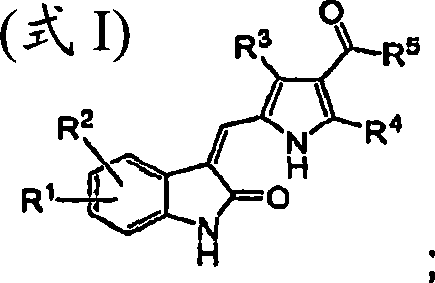
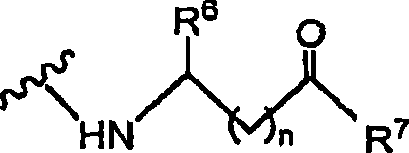
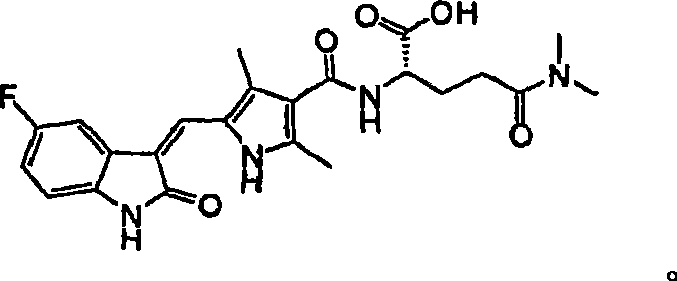
Amino acid derivatives of indolinone based protein kinase inhibitors
An amino and alkyl technology, applied in the field of protein kinase inhibitors, can solve the problems of weakening, poor water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-23
[0029] Examples 1-23: General scheme:
[0030]
[0031] Scenario 2
[0032] The synthesis of the starting HATU ester (1-1) is shown in Scheme 1. To prepare the free carboxylic acid 1-2, the unprotected amino acid (1.0 equiv) was added to a solution of 1-1 (1.0 equiv) and DIEA (1.5 equiv) in DMF as shown in Scheme 2. After stirring the solution overnight at 25°C, LC-MS showed complete formation of 1-2 with no starting material remaining. Amides 1-3 were prepared directly from this solution in the next step. Thus, the amine (2 equiv), HATU (1.0 mmol) and DIEA (1 equiv) were added to the solution. After stirring at 25°C for 2 hours, the reaction was found to be complete according to LC-MS analysis. The reaction solution was directly subjected to preparative HPLC to obtain pure amide products 1-3, which were subsequently characterized by LC-MS and NMR spectroscopy.
Embodiment 1
[0033] Example 1. Preparation of 5-[5-fluoro-2-oxo-1,2-dihydro-indole-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3 -Formic acid (2-dimethylcarbamoyl-propyl)-amide
[0034]
[0035] Preparative HPLC from 52 mg of starting material (active ester 1-1) gave 50 mg of the title compound (96%). LC-MS: singlet at 254nm, MH + Calculate C 22 h 25 FN 4 o 3 : 413, get value: 413.
[0036] 1 H-NMR (DMSO-d 6, 400MHz), δ13.68(s, 1H), 10.89(s, 1H), 7.76(dd, J=2.4Hz, 9.6Hz, 1H), 7.71(s, 1H), 7.68(t, J=5.6Hz , 1H), 6.93(m, 1H), 6.84(dd, J=4.4Hz, 8.4Hz, 1H), 3.31(m, 1H), 3.16(m, 2H), 3.05(s, 3H), 2.84(s , 3H), 2.41(s, 3H), 2.39(s, 3H), 1.03(d, J=6.8Hz, 3H).
Embodiment 25-
[0037] Example 2.5-[5-fluoro-2-oxo-1,2-dihydro-indole-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid ( 2-Methyl-3-(morpholin-4-yl)-3-oxo-propyl)-amide
[0038]
[0039] Preparative HPLC from 52 mg of starting material (active ester) gave 56 mg of the title compound (98%). LC-MS: singlet at 254nm, MH + Calculate C 24 h 27 f 2 N 4 o 4 : 455, get value: 455.
[0040] 1 H-NMR (DMSO-d 6 , 400MHz), δ13.68(s, 1H), 10.89(s, 1H), 7.75(dd, J=2.4Hz, 9.2Hz, 1H), 7.71(s, 1H), 7.67(t, J=5.6Hz , 1H), 6.92(m, 1H), 6.83(dd, J=4.8Hz, 8.4Hz, 1H), 3.55(m, 7H), 3.41(m, 1H), 3.35(m, 1H), 3.22(m , 1H), 3.12(m, 1H), 2.42(s, 3H), 2.40(s, 3H), 1.04(d, J=7.2Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com