Injection novel medicine for treating systematic lupus erythematosus
A lupus erythematosus and injection technology, applied in allergic diseases, inorganic active ingredients, organic active ingredients, etc., can solve problems such as large side effects and can not be used for a long time, and achieve the effect of strong anti-systemic lupus erythematosus effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
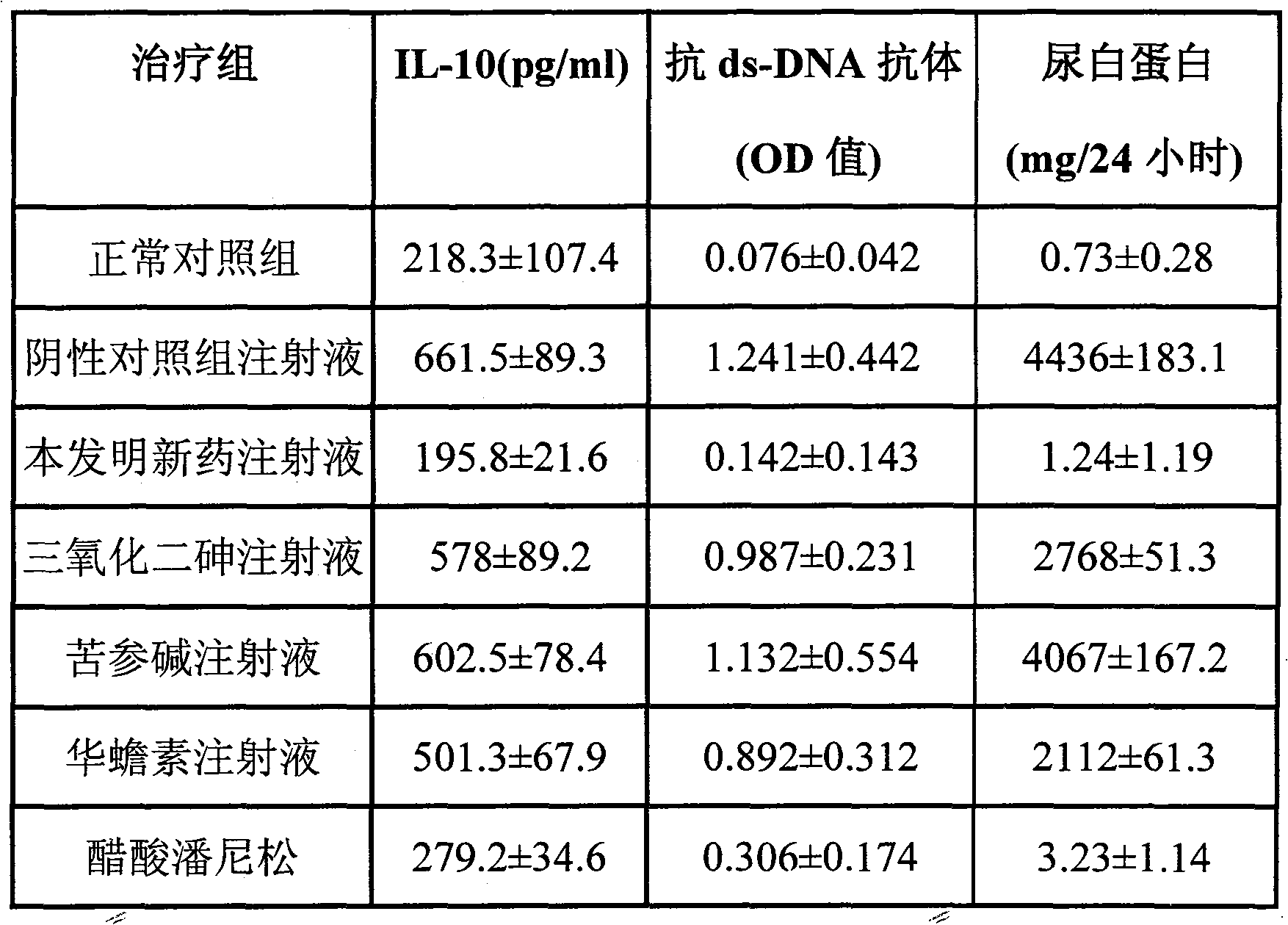
[0020] Dissolve 1 mg of arsenic trioxide in normal saline first, then add 40 mg of cinobufacin, stir to dissolve; finally add 40 mg of matrine and 1 mg of borneol, stir evenly to make the color into a light yellow liquid and sterilize it. finished product.
[0021] In the injection of this embodiment, every milliliter contains 1 milligram of arsenic trioxide, 40 milligrams of matrine, 40 milligrams of cinobufacin (containing serotonin at a concentration of 18 micrograms / ml or containing bufocin at a concentration of 25 micrograms / ml) and 1 mg borneol.
Embodiment 2
[0023] Dissolve 5 mg of arsenic trioxide in normal saline first, then add 50 mg of cinobufacin, stir to dissolve; finally add 50 mg of matrine and 5 mg of borneol, stir evenly to make the color into a light yellow liquid and sterilize it. finished product.
[0024] The injection in this example contains 5 mg of arsenic trioxide, 50 mg of matrine, 50 mg of cinobufacin and 5 mg of borneol per milliliter.
[0025] In order to further illustrate the outstanding effect of the injection of the present invention, the prepared single injection is compared with the injection of the present invention to prepare a single arsenic trioxide injection: dissolve arsenic trioxide (white color) with physiological saline, and each milliliter of arsenic trioxide injection contains 1 mg Arsenic trioxide.
[0026] Preparation of matrine oral liquid: dissolve matrine with normal saline, and each milliliter of matrine injection contains 1 mg of matrine.
[0027] Preparation of cinobufacin oral solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com