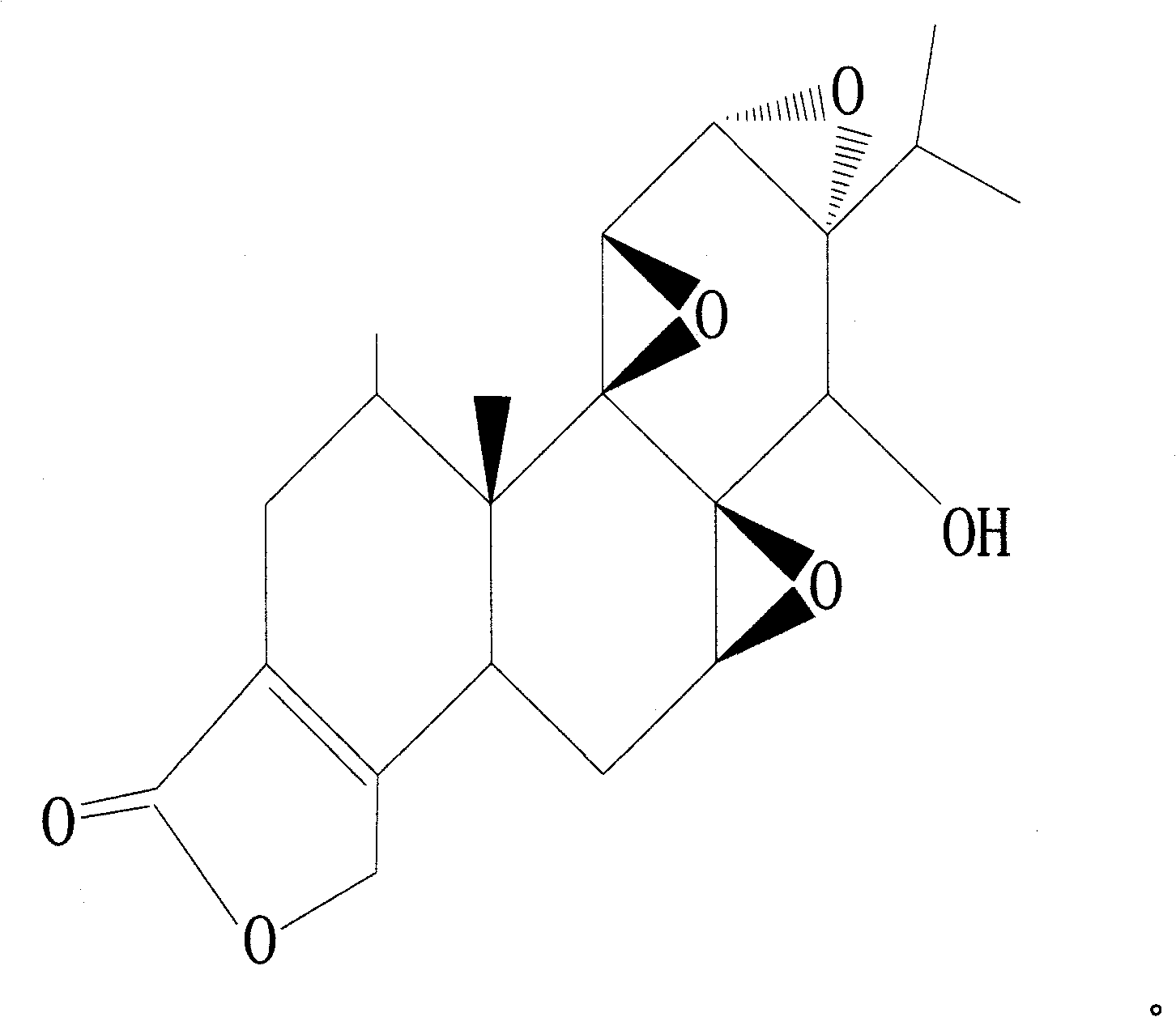
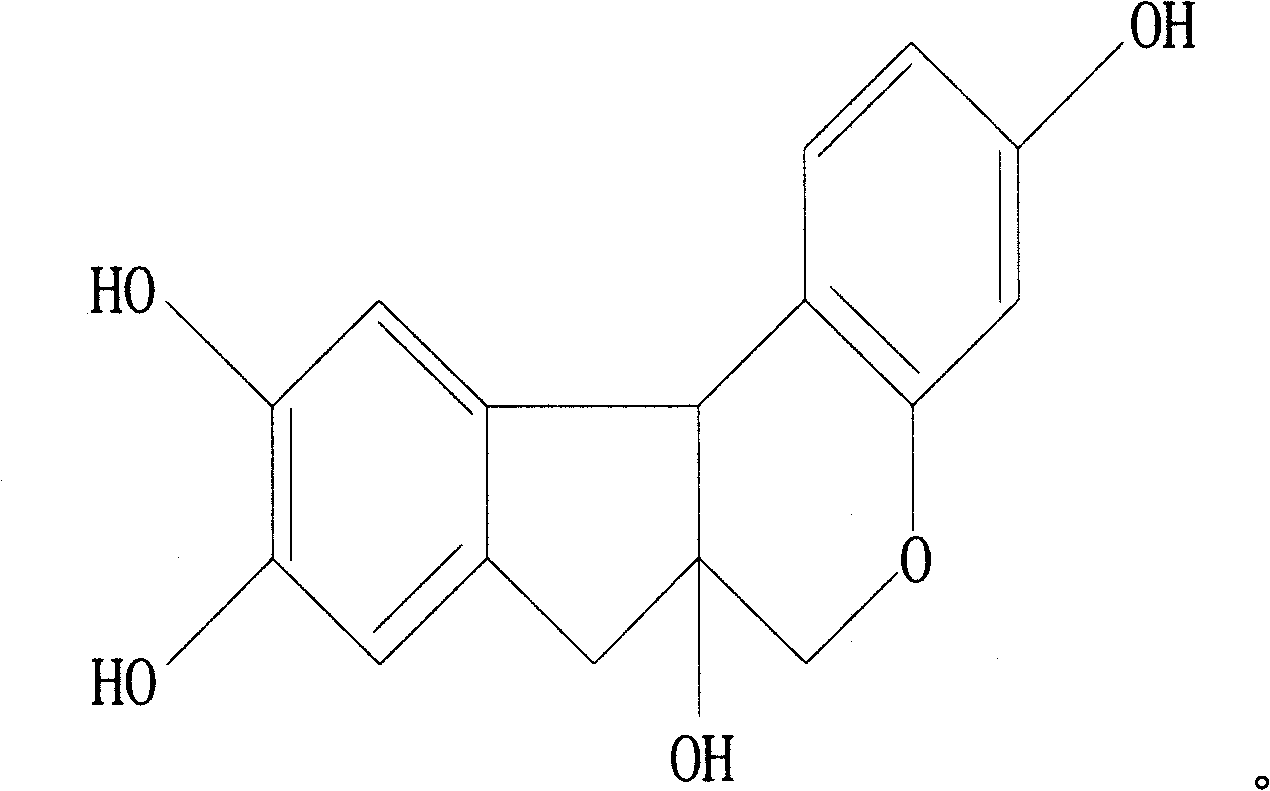
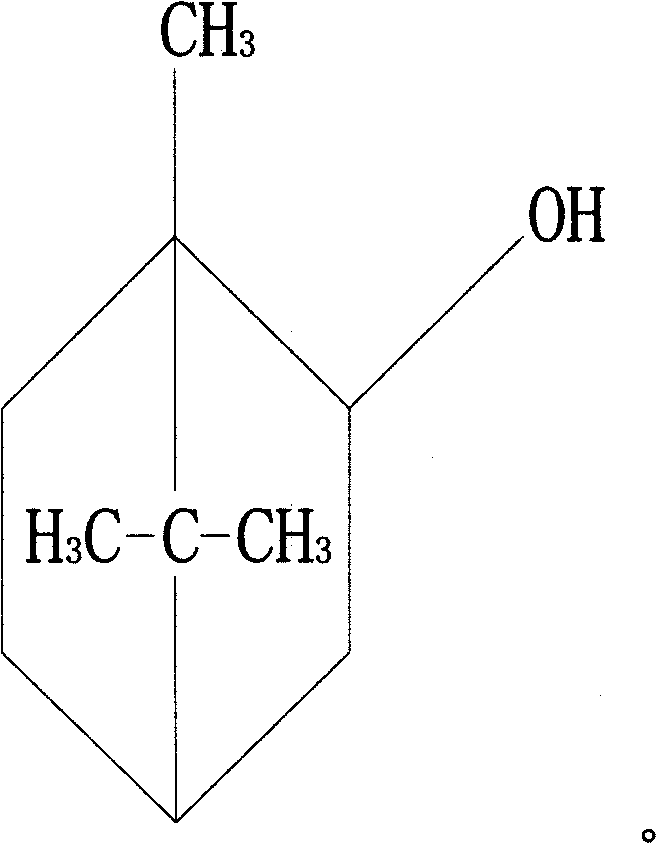
Novel anti-systematic erythema lupus medicine
A systemic technology for lupus erythematosus, applied in the field of medicine, can solve the problems of large side effects and impossibility of long-term use, and achieve the effect of small side effects, low cost and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Accurately weigh 1 mg of triptolide, 2200 mg of brazilianin, 799 mg of borneol and 7000 mg of polyethylene glycol 4000, place them in a heating container and heat while stirring, and heat to a temperature of 125°C to obtain the drug containing Melt or emulsion or suspension of dry powder and matrix.
[0036] Prepare dripping pills: adopt advanced dropping pill machine (TZDW-1 type dropping pill machine produced by Beijing Changzheng Tianmin High-tech Co., Ltd.), and adjust the temperature control system of the dropping pill machine, so that the dripper temperature of the dropping pill machine is heated and maintained At (50°C to 90°C), the temperature of the condensing agent is cooled and maintained at (4°C to -5°C). When the temperature of the condensing agent in the dripper of the dripping machine and the coagulation column is stable at the above required temperature state, the melt or emulsion or suspension containing the above-mentioned dry powder of the drug and th...
Embodiment 2
[0040] Accurately weigh 1 mg of triptolide, 340 mg of brazilianin, 100 mg of borneol and 2000 mg of polyethylene glycol 2000, place them in a heating container and heat while stirring, and heat to a temperature of 125°C to obtain the drug containing Melt or emulsion or suspension of dry powder and matrix.
[0041] Prepare dripping pills: adopt advanced dropping pill machine (TZDW-1 type dropping pill machine produced by Beijing Changzheng Tianmin High-tech Co., Ltd.), and adjust the temperature control system of the dropping pill machine, so that the dripper temperature of the dropping pill machine is heated and maintained At (50°C to 90°C), the temperature of the condensing agent is cooled and maintained at (4°C to -5°C). When the temperature of the condensing agent in the dripper of the dripping machine and the coagulation column is stable at the above required temperature state, the melt or emulsion or suspension containing the above-mentioned dry powder of the drug and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com