Use of gpr119 receptor agonists for increasing bone mass and for treating osteoporosis, and combination therapy relating thereto
An osteoporosis, agonist technology, applied in the field of GPR119 receptor agonists, can solve the problems of lack of oral bioavailability and affecting patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
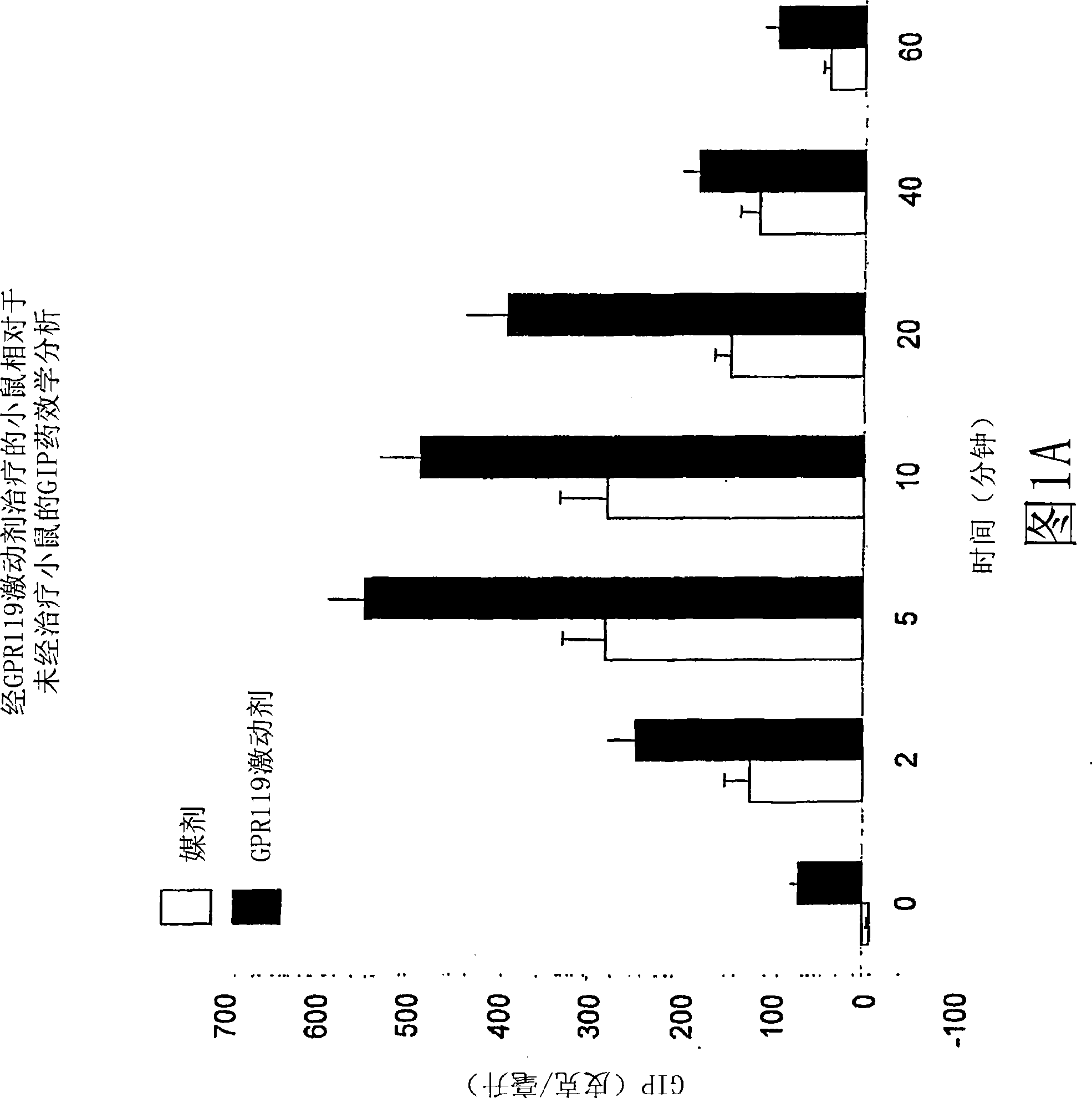
[0922] Example 1: Pharmacodynamic analysis of the effect of administration of GPR119 agonists on blood GIP levels in wild-type mice
[0923] A. C57blk / 6 male mice were fasted for 18 hours and randomly assigned to 14 groups, n=6 for each group. With vehicle (PET; 80% PEG400, 10% ethanol, 10% Tween80) or with 20 mg / kg of GPR119 agonist according to the invention (compound 1Z; (2-fluoro-4-methanesulfonyl-phenyl) -{6-[4-(3-isopropyl-[1,2,4]oxadiazol-5-yl)-piperidin-1-yl]-5-nitro-pyrimidin-4-yl}- amines) were administered orally to mice (as shown in Figure 1A). At 30 minutes post-treatment, a 3 g / kg glucose bolus was delivered orally, and at 0 minutes (no glucose bolus), 2 minutes, 5 minutes, 10 minutes, 20 minutes, 40 minutes, and 60 minutes after the glucose bolus. Plasma was collected at 1 min. By using the Rodent GIP ELISA Kit [Rat / Mouse Gastric Inhibitory Polypeptide (Total) ELISA, Catalog No. EZRMGIP-55K] purchased from Linco Research Laboratory (Linco Research Laboratory)...
example 2
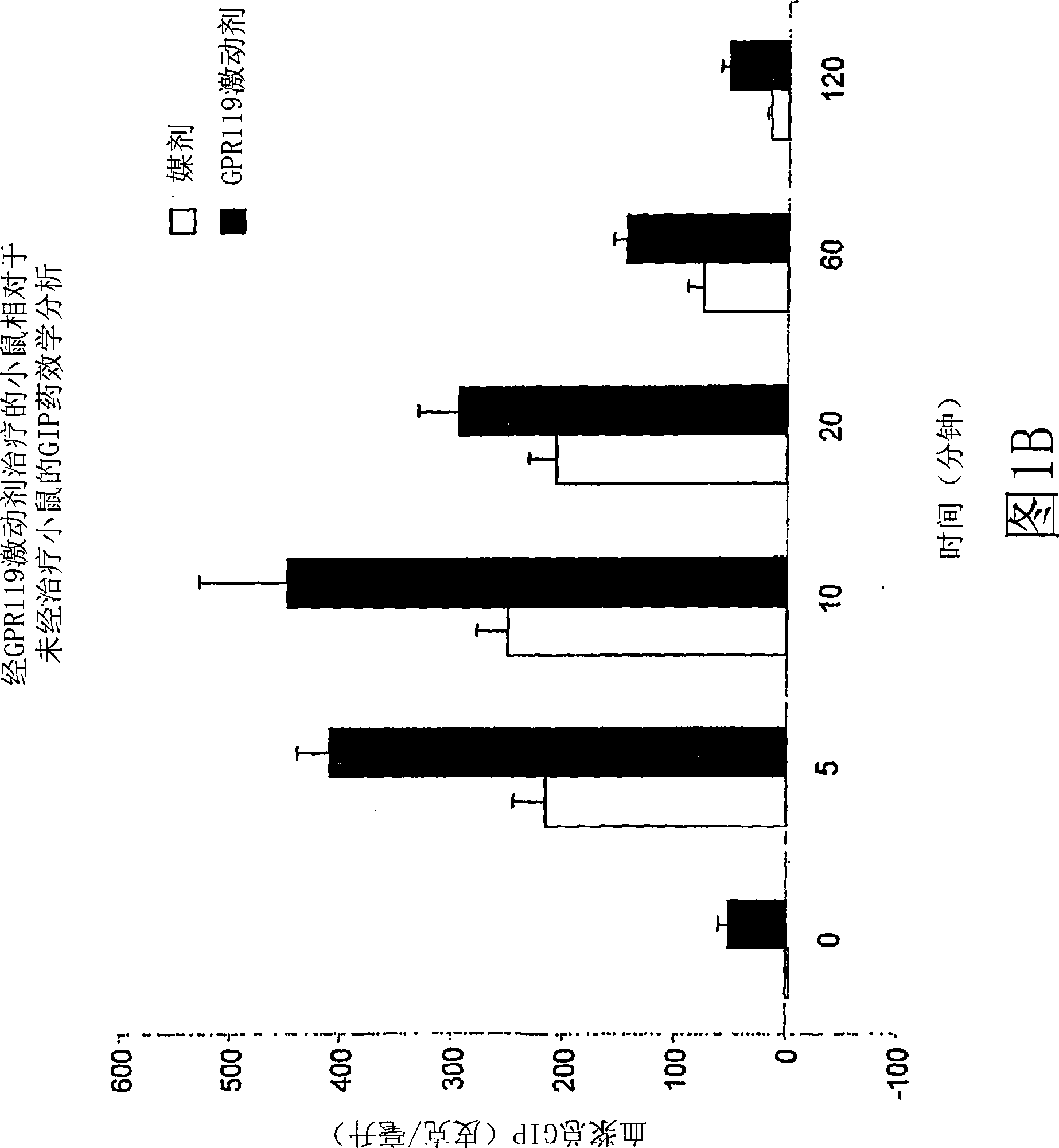
[0926] Example 2: Effect of Administration of a GPR119 Agonist on Blood GIP Levels in GPR119 Deficient (Knockout) Mice Compared to Wild Type Mice
[0927]A. GPR119-null male mice and wild-type littermates were fasted for 18 hours. With vehicle (PET; 80% PEG400, 10% ethanol, 10% Tween80) or with 20 mg / kg of GPR119 agonist according to the invention (compound 1Z; (2-fluoro-4-methanesulfonyl-phenyl) -{6-[4-(3-isopropyl-[1,2,4]oxadiazol-5-yl)-piperidin-1-yl]-5-nitro-pyrimidin-4-yl}- amines) were administered orally to mice (as indicated (n=5)). At 30 minutes post-treatment, blood (100 microliters) was collected via the retro-orbital vein of the eye (time 0), followed by administration of a 3 g / kg glucose bolus (po). Another blood sample (100 microliters) was collected 5 minutes after the glucose delivery (time 5 minutes). Plasma was collected after centrifugation and analyzed by using the Rodent GIP ELISA Kit [Rat / Mouse Gastric Inhibitory Polypeptide (Total) ELISA, Cat. No. EZR...
example 3
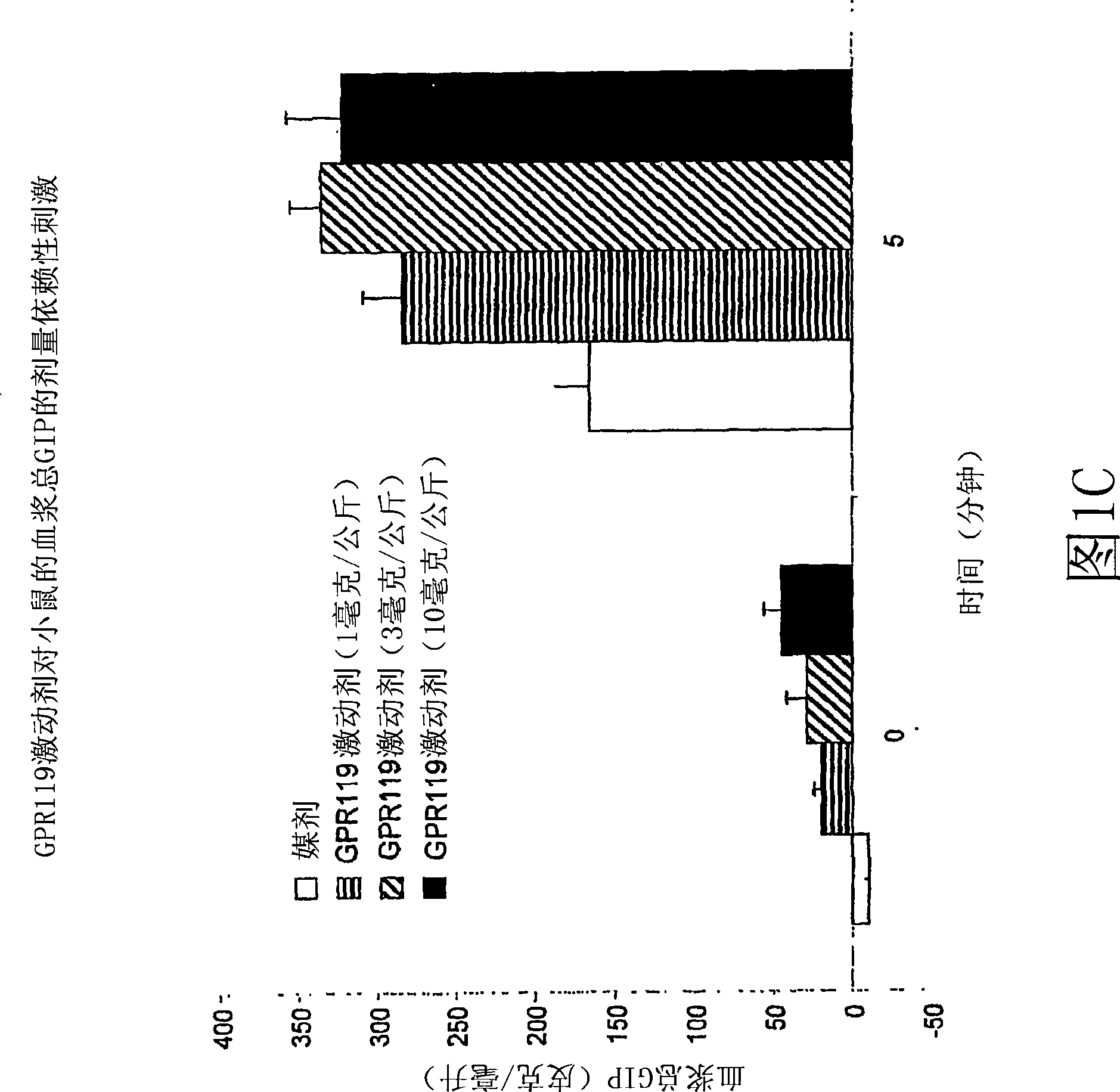
[0929] Example 3: Effect of Administration of a GPR119 Agonist in Combination with a DPP-IV Inhibitor on Blood GIP Levels in Wild-Type Mice
[0930] Using the in vivo assays described below, it can be shown that an amount of a GPR119 agonist in combination with an amount of a DPP-IV inhibitor according to the invention increases the GIP content in the blood of an individual.
[0931] C57blk / 6 male mice were fasted for 18 hours and randomly assigned to 14 groups, n=6 for each group. Mice were orally dosed with vehicle (PET; 80% PEG400, 10% ethanol, 10% Tween80) or a combination of an amount of GPR119 agonist and an amount of DPP-IV inhibitor. The experimental group was similar to that of Example 1 above. Each of the combined GPR119 agonist and DPP-IV inhibitor is used in an amount between 0.001 mg / kg body weight and 100 mg / kg body weight. At 30 minutes post-treatment, a 3 g / kg glucose bolus was delivered orally, and at 0 minutes (no glucose bolus), 2 minutes, 5 minutes, 10 mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com