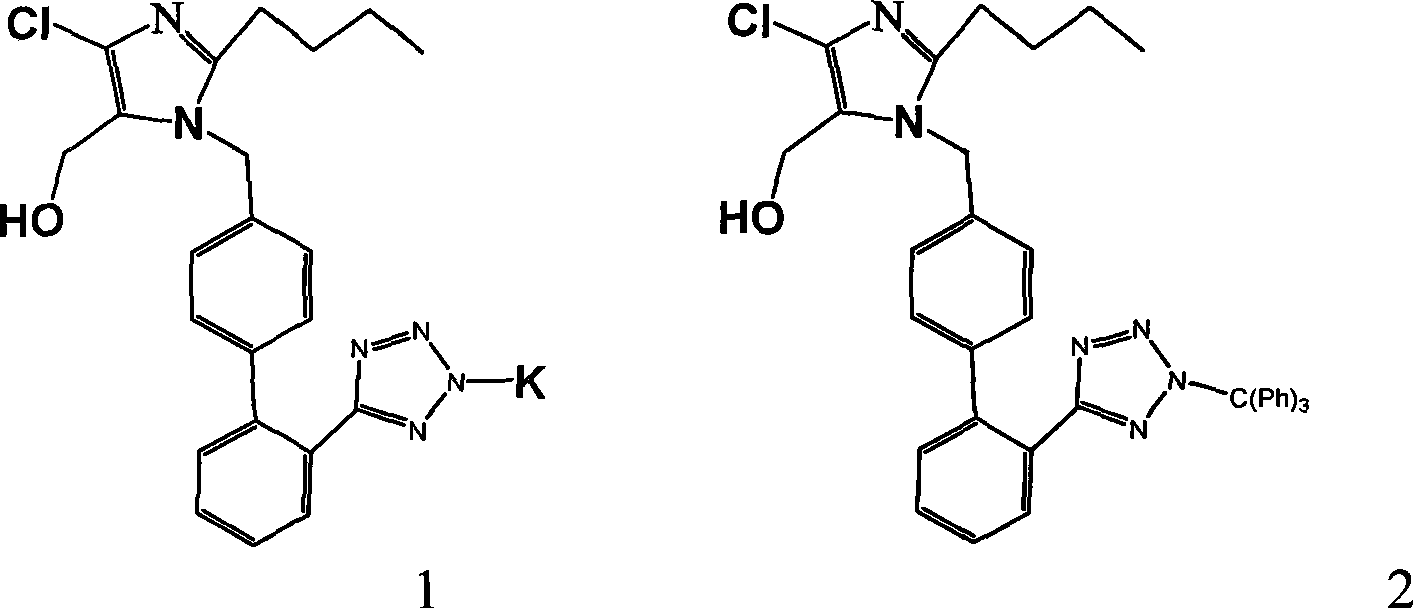
Novel preparation of losartan potassium
A technology of losartan potassium and n-butyl, applied in the field of drug synthesis, can solve the problems such as difficulty in obtaining raw materials, and achieve the effect of high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] step a
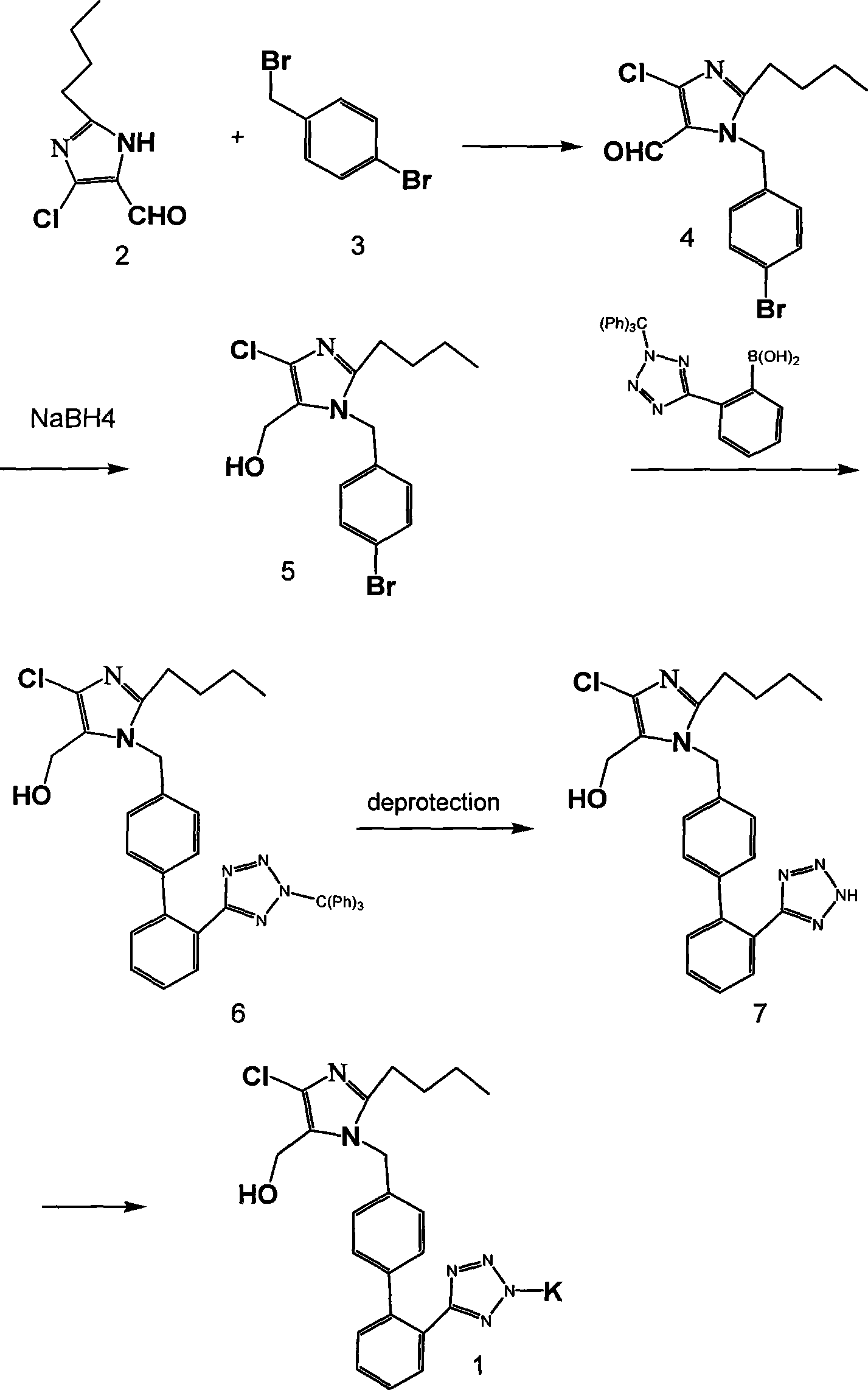
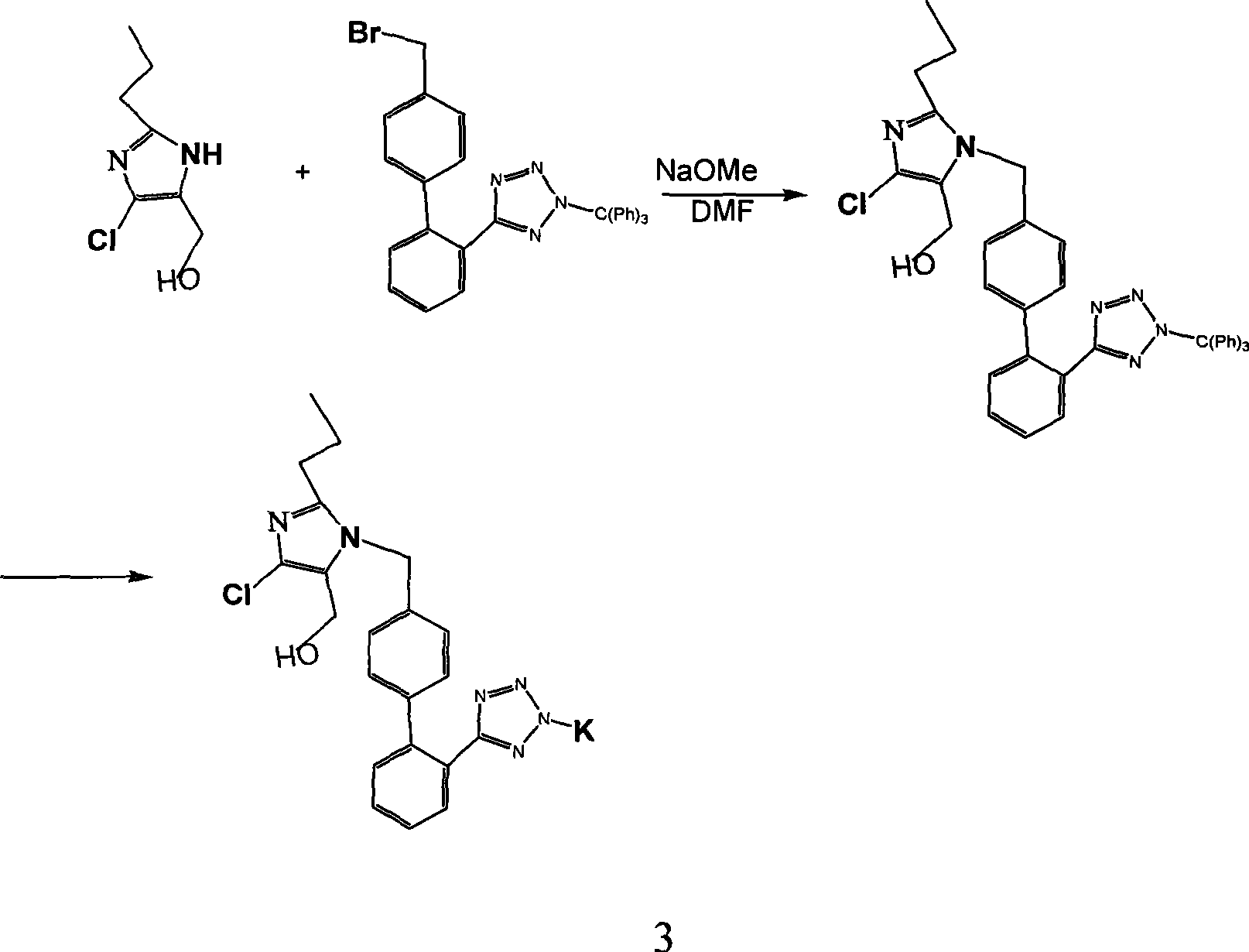
[0019] Preparation of 2-n-butyl-4-chloro-1-[4-bromo-benzyl]-1H-imido-5-formyl:
[0020] In a 1000ml four-neck flask equipped with a drying tube, a thermometer, a dropping funnel and mechanical stirring, add 2g of 2-n-butyl-4-chloro-1H-imido-5-formyl, dichloromethane, stir to dissolve, and cool down . At -10-0°C (eg -10°C, -5°C, -3°C, 0°C), add 2.5g of p-bromobenzyl bromide in dichloromethane dropwise; after addition, continue the reaction at 0-10°C After 4 to 5 hours, TLC showed that the raw material basically disappeared; adding 5% aqueous sodium bicarbonate solution for washing, washing with saturated brine, and removing dichloromethane from the organic phase under reduced pressure to obtain 2-n-butyl-4-chloro-1-[4 -Bromo-benzyl]-1H-imido-5-formyl 4.2 g. The crude product was directly carried on to the next step without further purification.
[0021] step b
[0022] Preparation of 2-n-butyl-4-chloro-1-[4-bromo-benzyl]-1H-midine-5-methanol:
[0023] The ...
Embodiment 2
[0034] step a
[0035] Preparation of 2-n-butyl-4-chloro-1-[4-bromo-benzyl]-1H-imido-5-formyl:
[0036] In a 1000ml four-necked flask equipped with a drying tube, a thermometer, a dropping funnel and mechanical stirring, add 2-n-butyl-4-chloro-1H-imido-5-formyl 2g, toluene, diisopropylethylamine 3ml, stir to dissolve, and cool down. At -10-0°C, add 2.5g of toluene solution of p-bromobenzyl bromide dropwise; after the addition, continue to react for 6 hours at 0-5°C, TLC shows that the raw materials basically disappear; add 5% aqueous sodium bicarbonate to wash, Wash with saturated brine, and remove toluene from the organic phase under reduced pressure to obtain 4.2 g of 2-n-butyl-4-chloro-1-[4-bromo-benzyl]-1H-imido-5-formyl. The crude product was directly carried on to the next step without further purification.
[0037] step b
[0038] Preparation of 2-n-butyl-4-chloro-1-[4-bromo-benzyl]-1H-midine-5-methanol:
[0039] The product of step a was dissolved in 150 ml of dic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com