Pharmaceutical composition containing a tetrahydrofolic acid
A technology of tetrahydrofolate and composition, which is applied in the direction of drug combination, medical preparation containing active ingredients, pharmaceutical formula, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1 - Direct Compression; Microcrystalline Cellulose
[0099] 80 mg tablet cores with the following composition are prepared by direct compression:
[0100]
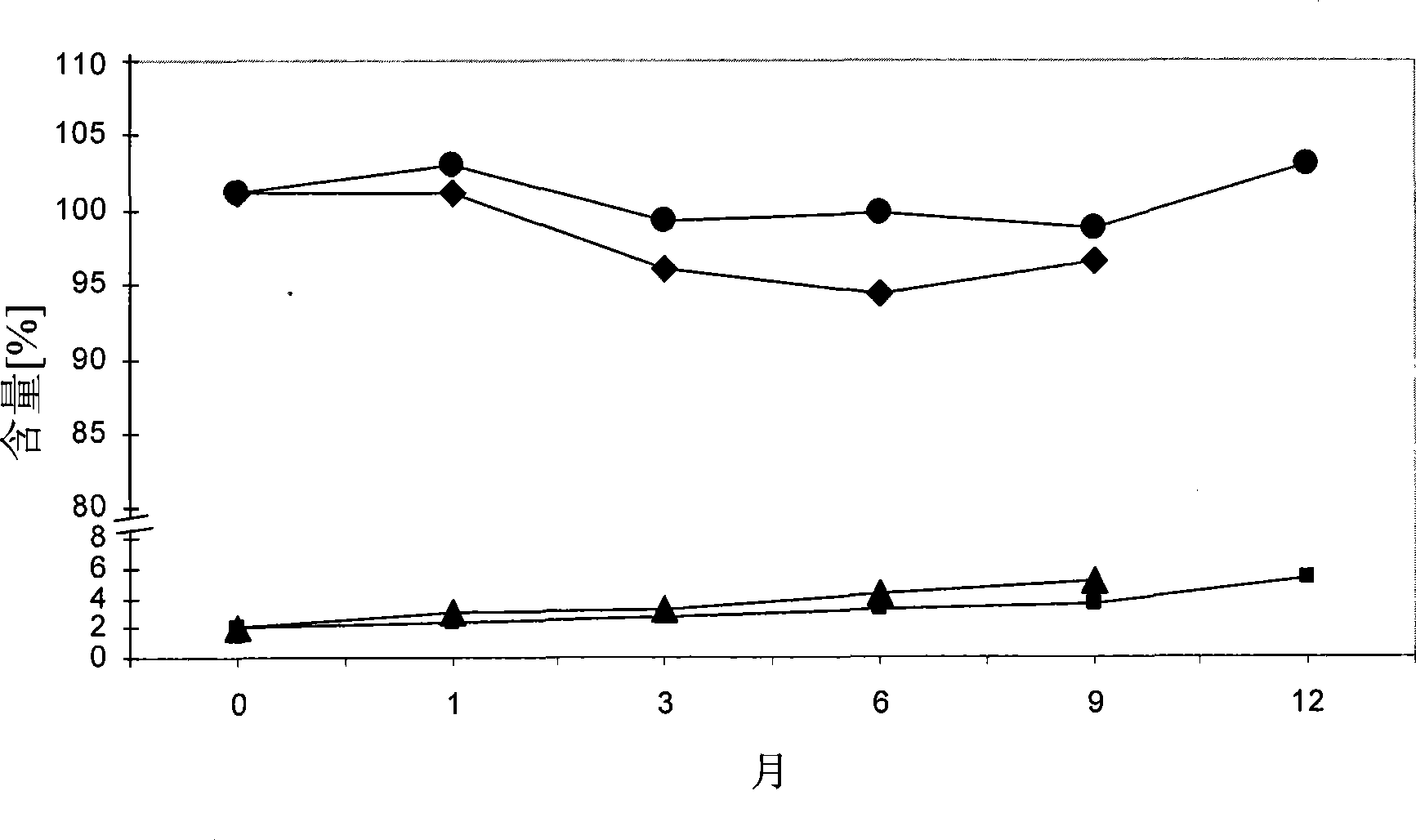
[0101] To test the stability of calcium 5-methyl-(6S)-tetrahydrofolate during storage under various conditions. The following stability data were obtained upon storage at 25°C / 60%RH and 40°C / 75%RH respectively (see Tables 1 and 2 below). Stability was tested in open and closed containers.
[0102] Table 1: Percentage of total degradation products
[0103]
[0104] Table 2: Amount (%)
[0105]
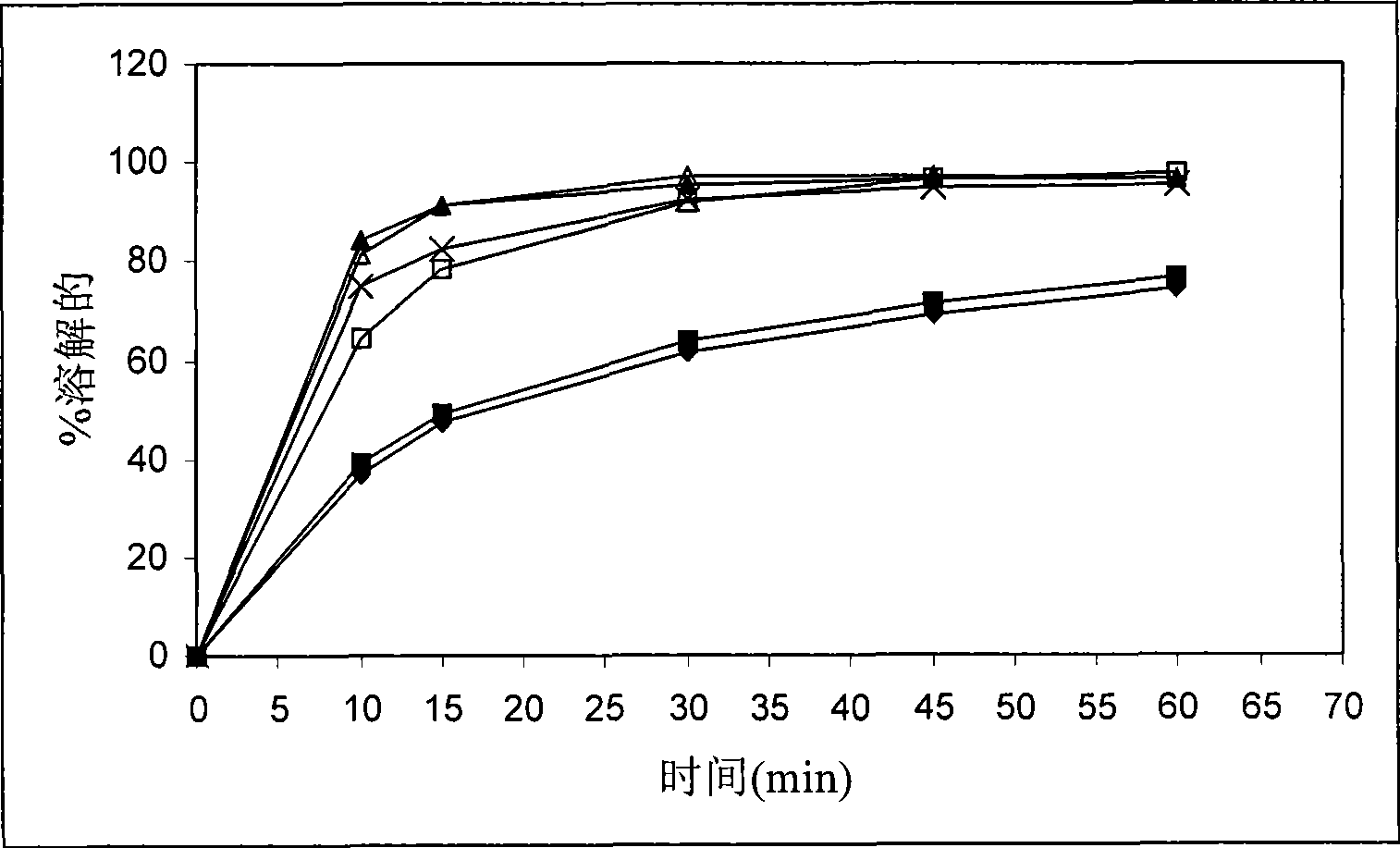
[0106] It can be seen that satisfactory stability of calcium 5-methyl-(6S)-tetrahydrofolate was obtained at 25°C even under conditions that allowed the tablets to be exposed to open air. In addition, satisfactory stability was obtained at 40°C (closed container), while 5-methyl-(6S)-tetrahydrofolate calcium was seen when the tablets were stored at 40°C while being exposed to open air. Significantly degr...
Embodiment 2
[0108] Embodiment 2-direct compression tablet; (Lactose Monohydrate / Cellulose Powder)
[0109] 80 mg tablet cores with the following composition are prepared by direct compression:
[0110]
[0111] To test the stability of calcium 5-methyl-(6S)-tetrahydrofolate during storage under various conditions. The following stability data were obtained upon storage at 25°C / 60%RH and 40°C / 75%RH respectively (see Tables 3 and 4 below). Stability was tested in open and closed containers.
[0112] Table 3: Sum of decomposition products (%)
[0113]
[0114] Table 4: percentage amount
[0115]
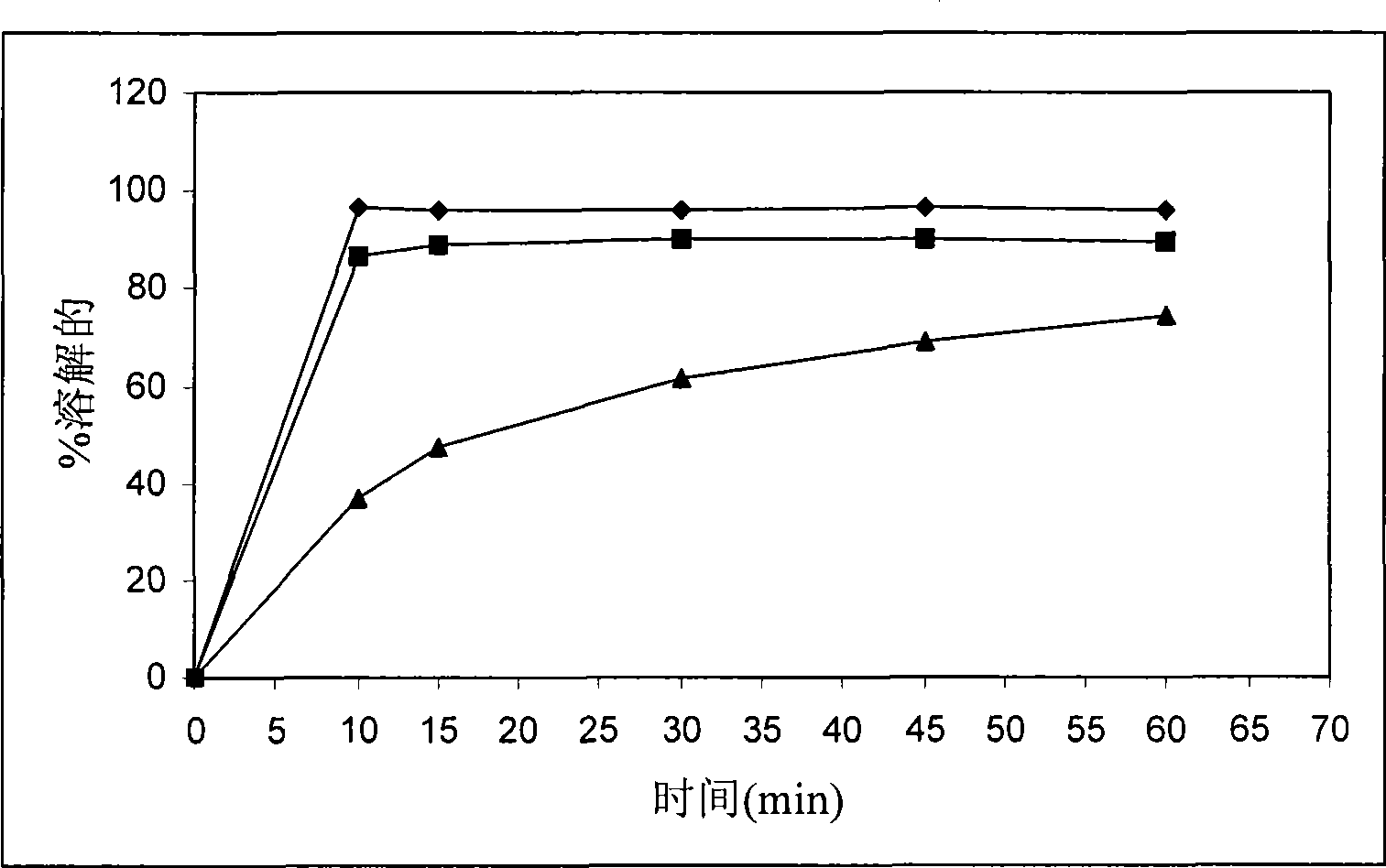
[0116] It can be seen that satisfactory stability of calcium 5-methyl-(6S)-tetrahydrofolate was obtained at 25°C. However, like Example 1, the dissolution rate of drospirenone was unsatisfactorily slow.
Embodiment 3
[0117] Embodiment 3 - direct compression tablet; (lactose monohydrate)
[0118] 80 mg tablet cores with the following composition are prepared by direct compression:
[0119]
[0120] The stability of calcium 5-methyl-(6S)-tetrahydrofolate was found to be unsatisfactory when stored at 25°C / 60%RH and 40°C / 75%RH, respectively. Like Examples 1 and 2, the dissolution rate of drospirenone was unsatisfactorily slow.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com