Preparation method of drospirenone
A drospirenone and dihydroxy technology, applied in the field of preparation of drospirenone, can solve the problems of low product yield, high production cost, poor product quality and the like, and achieve high yield and purity, low cost and short preparation steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
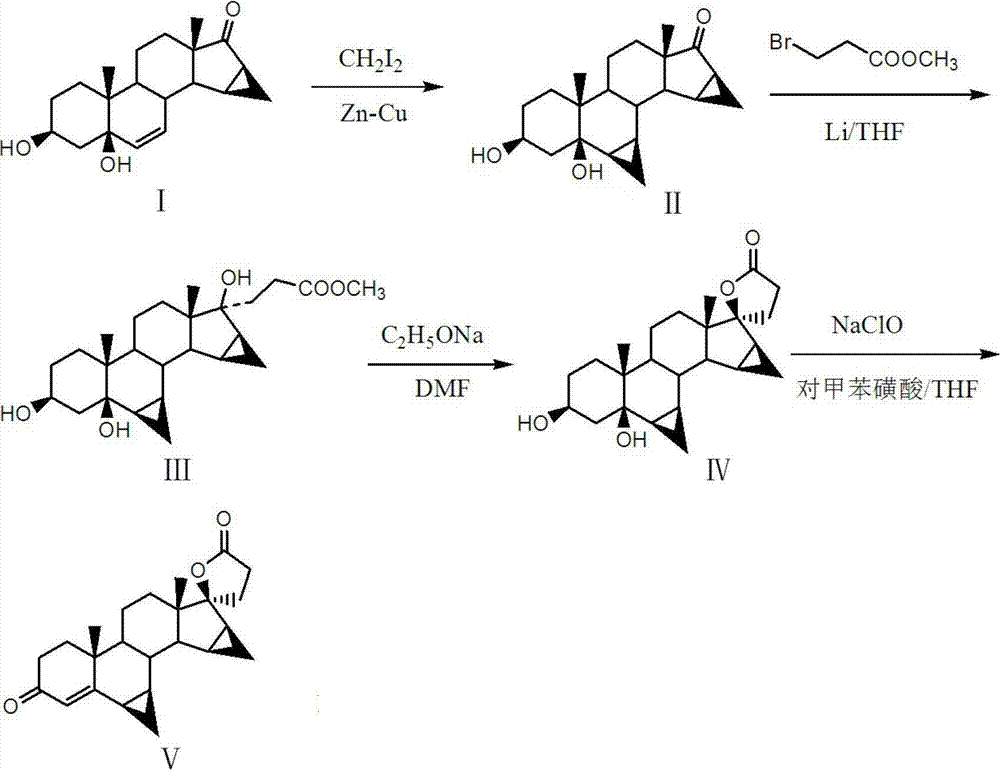
[0034] (1) Preparation of 3β,5-dihydroxy-6β,7β,15β,16β-dimethylene-5β-androst-17-one
[0035]Add 220ml of THF, 13g of cuprous chloride and 58g of zinc powder into the reaction flask, heat to 65°C and keep reflux reaction for 4h, then add 14.5g of 3β, 5-dihydroxy-15β, 16β after cooling to room temperature -methylene-5β-androst-6-en-17-one, then dropwise add 63g diiodomethane to the reactor, control the rate of addition to keep the reaction temperature at room temperature, and keep the temperature until TLC after the addition of diiodomethane The test showed that the substrate point completely disappeared, and 60g of acetic acid solution with a mass concentration of 36% was added dropwise, and the precipitated solid was collected and dried to obtain 13.1g of 3β,5-dihydroxy-6β,7β,15β,16β-dimethylene Base-5β-androst-17-one. TLC: petroleum ether: ethyl acetate = 1:2.
[0036] (2) Preparation of 3β,5-dihydroxy-6β,7β,15β,16β-dimethylene-5β-androst-17β-hydroxy-21-carboxylic acid met...
Embodiment 2
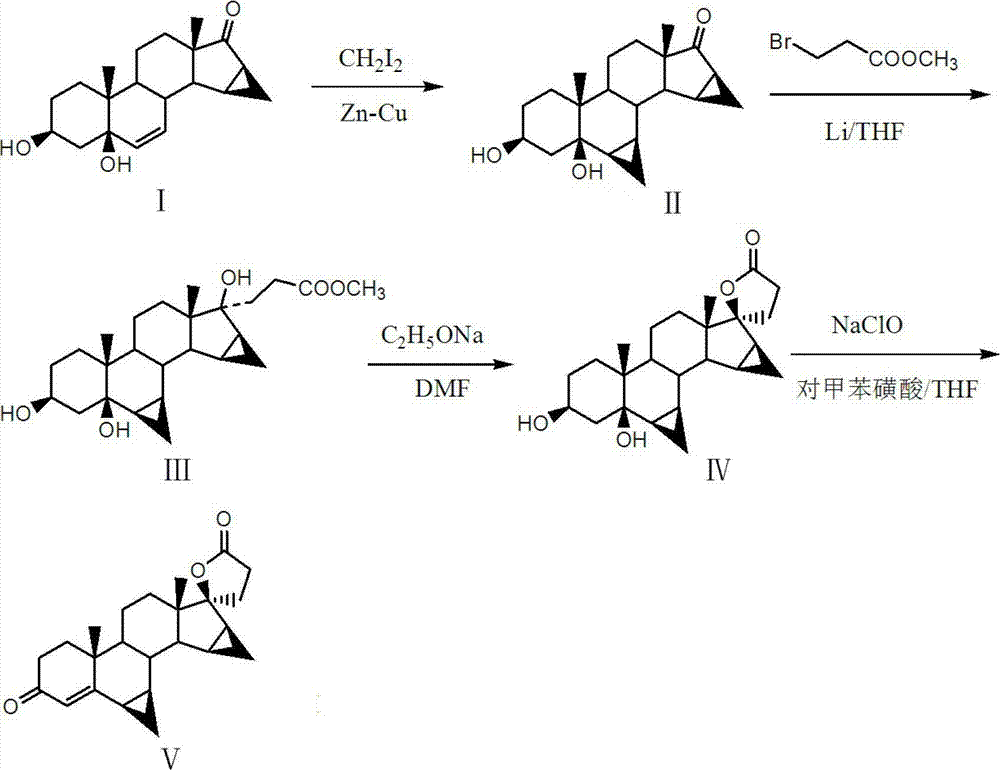
[0043] The other steps of this example are the same as in Example 1, except that the 3β,5-dihydroxy-6β,7β,15β,16β-dimethylene-5β-androst-17-one in step (1) preparation:
[0044] Add 225ml of THF, 15g of cuprous chloride and 60g of zinc powder into the reaction flask, heat to 70°C and keep reflux reaction for 3h, then add 15g of 3β,5-dihydroxy-15β,16β- Methylene-5β-androst-6-en-17-one, then dropwise add 64g of diiodomethane to the reactor, control the rate of addition to keep the reaction temperature at room temperature, keep the reaction until TLC detection after the addition of diiodomethane is completed It shows that the substrate point has completely disappeared, and 64g of acetic acid solution with a mass concentration of 34% is added dropwise, and the precipitated solid is collected and dried to obtain 13.3g of 3β,5-dihydroxy-6β,7β,15β,16β-dimethylene -5β-androst-17-one. TLC: petroleum ether: ethyl acetate = 1:2.
Embodiment 3
[0046] The other steps of this example are the same as in Example 1, except that the 3β,5-dihydroxy-6β,7β,15β,16β-dimethylene-5β-androst-17-one in step (1) preparation:
[0047] Add 230ml of THF, 17g of cuprous chloride and 62g of zinc powder into the reaction flask, heat to 75°C and keep reflux reaction for 3.5h, then add 15.5g of 3β, 5-dihydroxy-15β after cooling to room temperature, 16β-methylene-5β-androst-6-en-17-one, then dropwise add 65g diiodomethane to the reactor, control the rate of addition so that the reaction temperature is maintained at room temperature, and keep the insulation reaction until the diiodomethane is added dropwise. TLC detection showed that the substrate point completely disappeared, and 65g of acetic acid solution with a mass concentration of 33% was added dropwise, and the precipitated solid was collected and dried to obtain 13.4g of 3β,5-dihydroxy-6β,7β,15β,16β-diethylene Methyl-5β-androst-17-one. TLC: petroleum ether: ethyl acetate = 1:2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com