Long term 24 hour intestinal administration of levodopa/carbidopa
A levodopa, 24-hour technology for neurological diseases, organic active ingredients, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
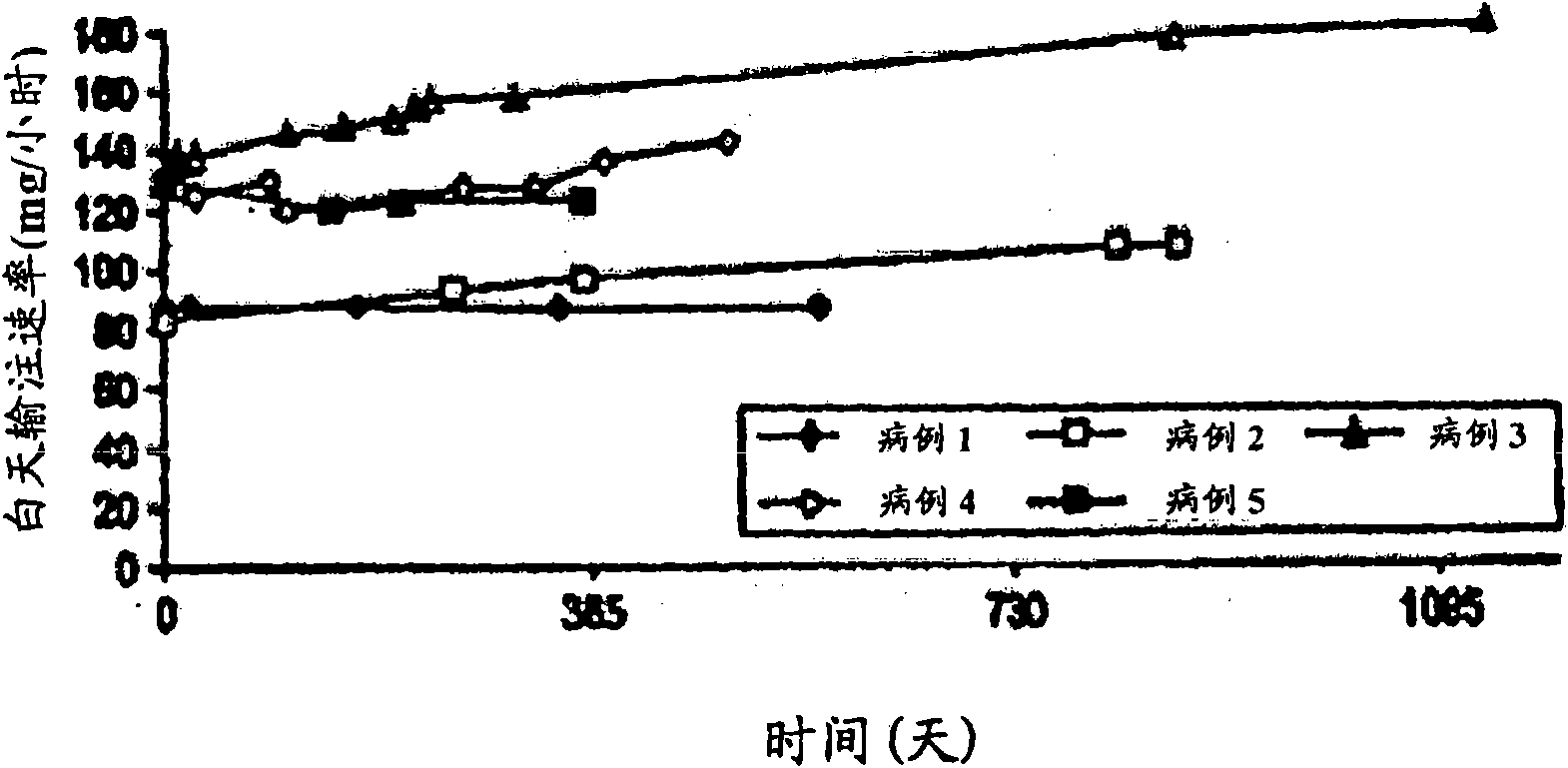
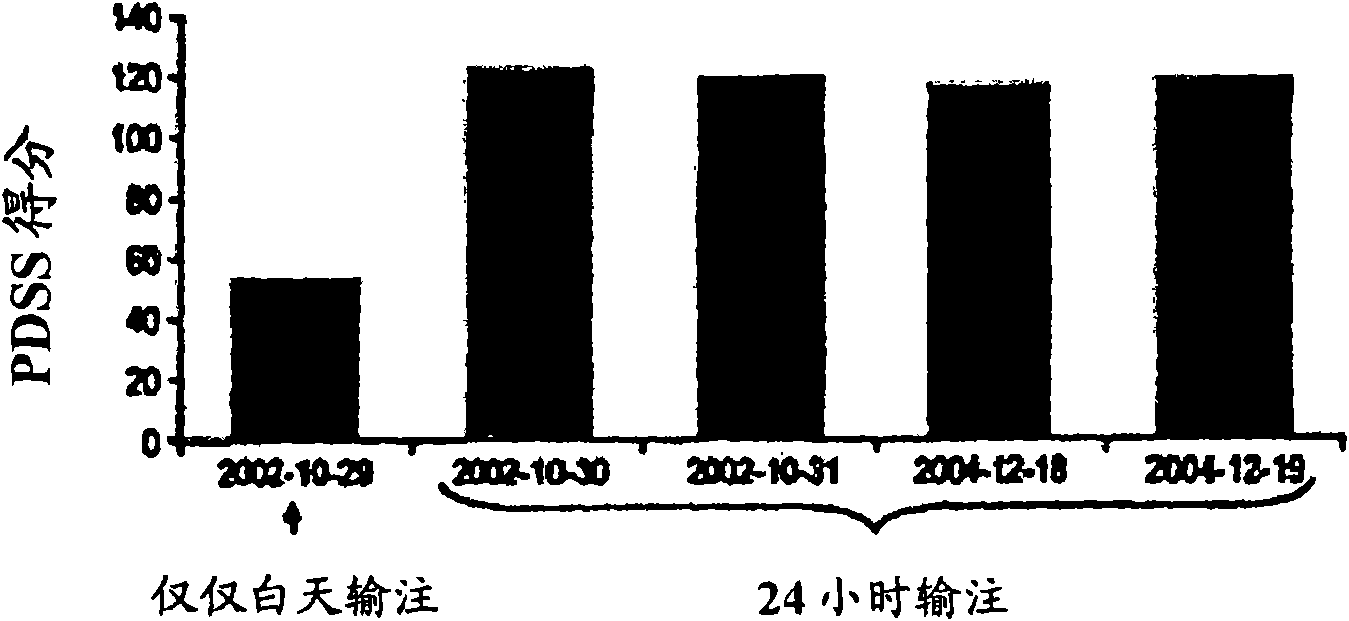
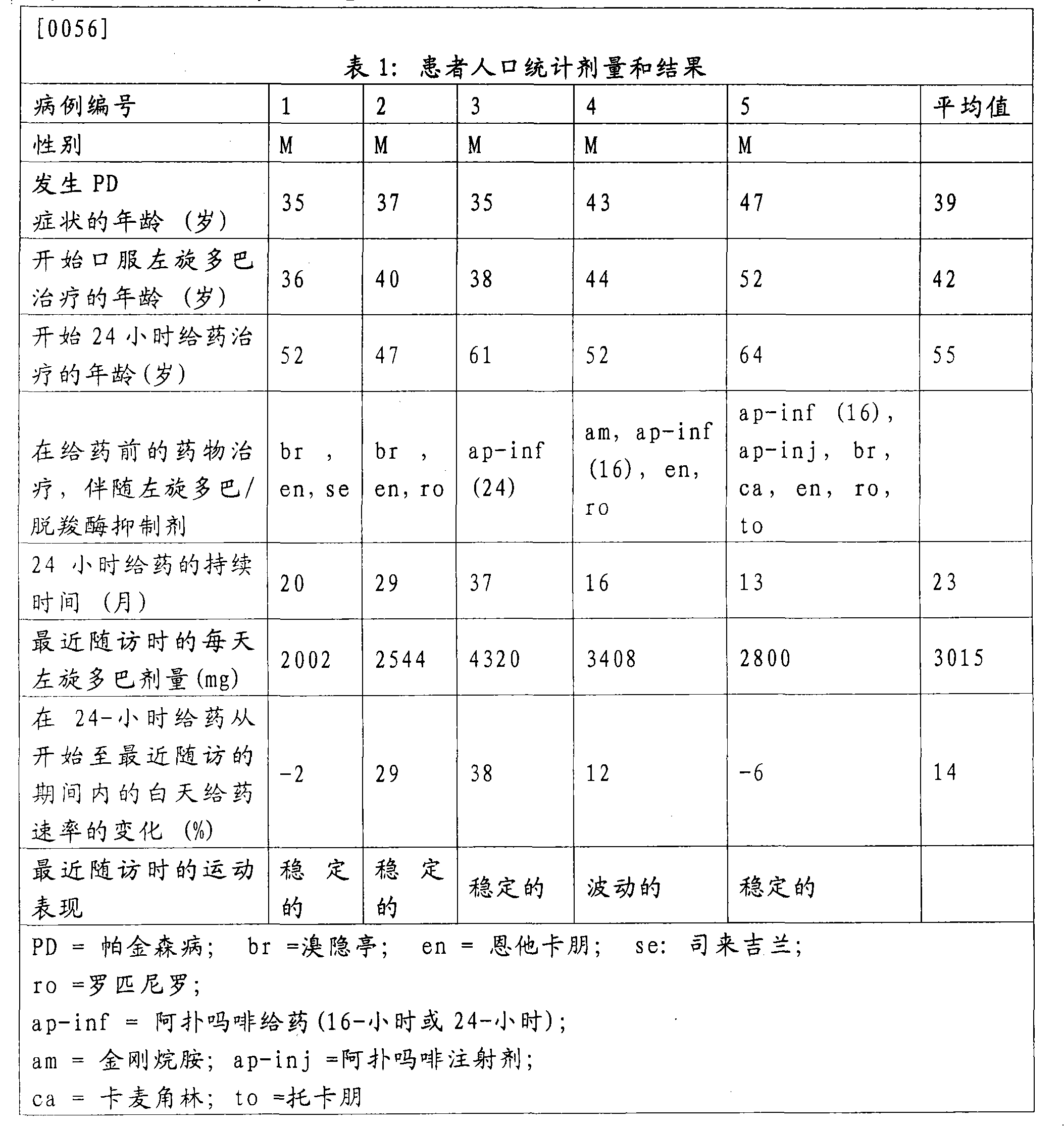
[0051] Most patients with PD suffer from sleep disorders. In advanced PD, dopaminergic drugs are sometimes used frequently at night to improve sleep. Because of concerns about tolerability and psychiatric side effects, levodopa was administered for 24 consecutive hours only in a very small number of patients and in a short period of time. Researched the 24-hour duodenal administration of levodopa / carbidopa ( ) Of 5 cases, lasting until 37 months.
[0052] method
[0053] The hospital chart was reviewed retrospectively in 5 PD patients who received a 4:1 weight ratio of levodopa / carbidopa in the gel for 24 hours ( ) Duodenal administration by enteral administration. The formulation is described in US Patent No. 5,635,213, which is incorporated into this application by reference. Record dosage, efficacy, sleep patterns and side effects.
[0054] For their health, the patients were treated with this regimen. Therefore, no prospective research protocol was used. In one case, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com