Repaglinide substantially free of dimer impurity
A dimer and impurity technology, applied in the field of preparation of repaglinide, can solve the problems of high reagent cost and low product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0110] According to another aspect of the present invention, the preparation method of the dimer impurity of formula IIa is provided, it comprises:
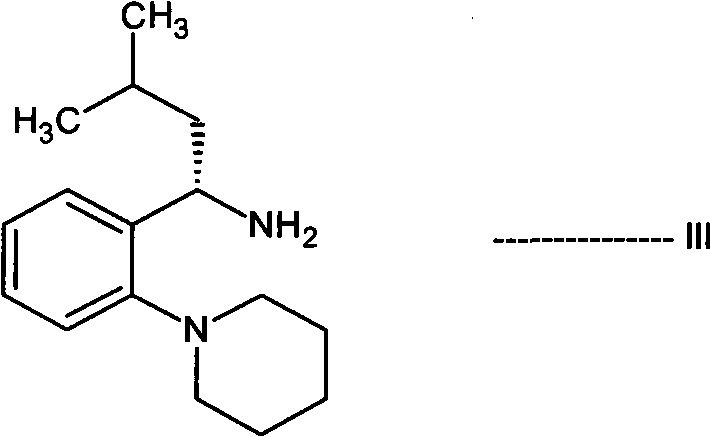
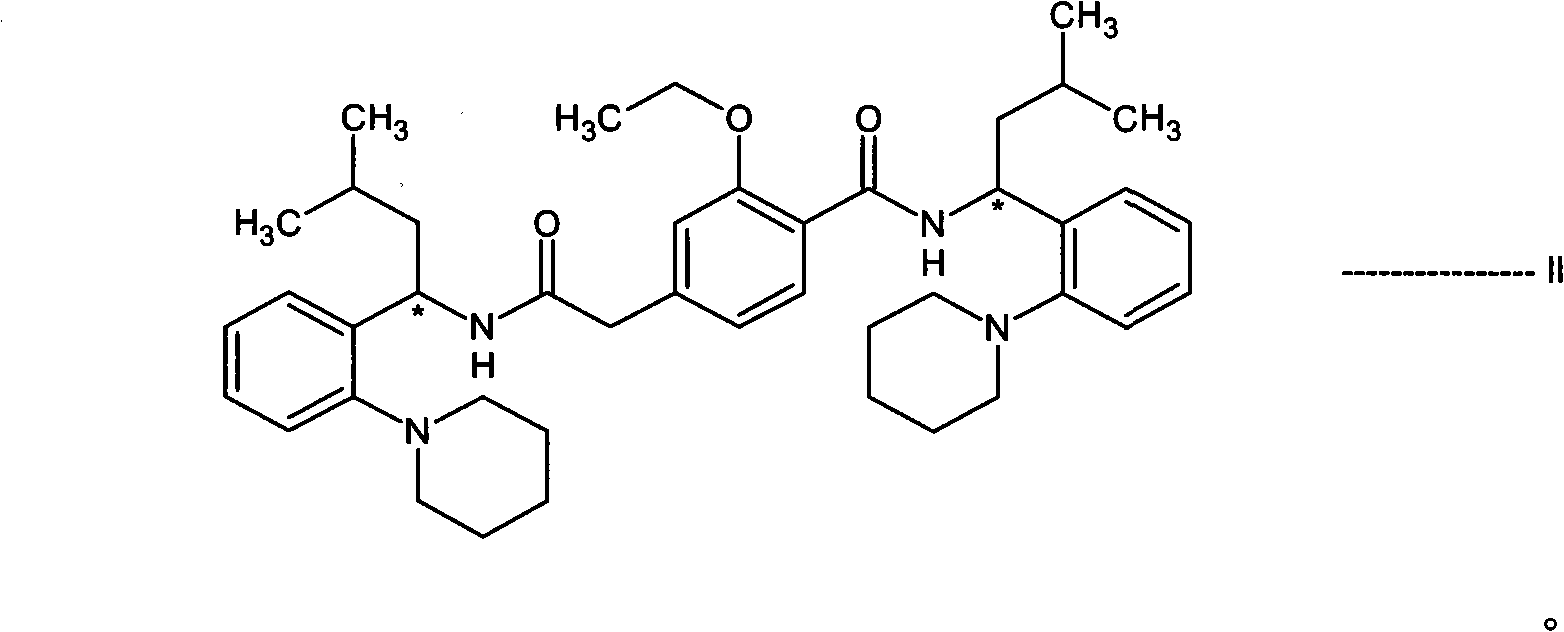
[0111] In the presence of a dehydrating agent selected from boric acid or boric acid derivatives and in a suitable solvent, (S)-3-methyl-1-(2-piperidinylphenyl)-1-butan having a structure of formula III The amine or its salt is reacted with 3-ethoxy-4-ethoxycarbonylphenylacetic acid or its salt having the structure of formula VI to produce IIa compound 2-ethoxy-N-[(1S)-3- Methyl-1-[2-(1-piperidinyl)phenyl]butyl]-4-[2-[[(1S)-3-methyl-1-[2-(1-piperidinyl) phenyl]butyl]amino]-2-oxoethyl]benzamide.
[0112]
[0113] Exemplary boronic acid derivatives include, but are not limited to: aryl or substituted arylboronic acids, such as phenylboronic acid, 2-chlorophenylboronic acid, 2-nitrophenylboronic acid, 3-nitrophenylboronic acid, 4- Nitrophenylboronic acid, 2-carboxyphenylboronic acid, 2-chloro-4-carboxyphenylboronic acid, 2-chlo...
Embodiment 1
[0181] Preparation method of pure repaglinide substantially free of dimer impurities
[0182] Crude repaglinide (15 g, dimer impurity content: 0.35%) was dissolved in toluene (90 mL) at 60-65°C. Cyclohexane (15 mL) was then added to the hot solution at 60-65°C. The solution was cooled slowly at 25-30°C and stirred for 1-2 hours. The precipitated product was filtered, washed with cyclohexane (30 ml), and then vacuum-dried at 50-55° C. for 6 hours to obtain 12.5 g of pure repaglinide (yield: 83.3%; dimerization as measured by HPLC). Bulk impurity content: 0.06%).
Embodiment 2
[0184] 2-Ethoxy-N-[(1S)-3-methyl-1-[2-(1-piperidinyl)phenyl]butyl]-4-[2-[[(1S)-3- Preparation of methyl-1-[2-(1-piperidinyl)phenyl]butyl]amino]-2-oxoethyl]benzamide (dimer impurity)
[0185]In a round bottom flask equipped with a Dean Stark condenser, (S)-3-methyl-1-(2-piperidinylphenyl)-1-butylamine (2.0 g, 0.00813 mol) was dissolved in toluene ( 50 mL), then 3-ethoxy-4-ethoxycarbonylphenylacetic acid (0.91 g, 0.00406 mol) and phenylboronic acid (0.099 g, 0.000813 mol) were added. The reaction mixture was refluxed for 16-18 hours. The reaction mixture was then cooled to 25-30°C and then filtered. The toluene layer was washed with water and 1% sodium bicarbonate solution. Then the toluene was distilled off completely. To the resulting residue was added hexane (20 mL) to precipitate a solid and stirred for 1 hour. The resulting solid was filtered and washed with hexane (10 mL). The crude product was purified by column chromatography to prepare 2-ethoxy-N-[(1S)-3-methyl-1-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com