Substituted piperazine N-ethyl sulfonamide derivative and preparation and application thereof
A technology of sulfonamide and derivatives, applied in the field of preparing antitumor drugs, can solve the problem of low selectivity of PKB
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
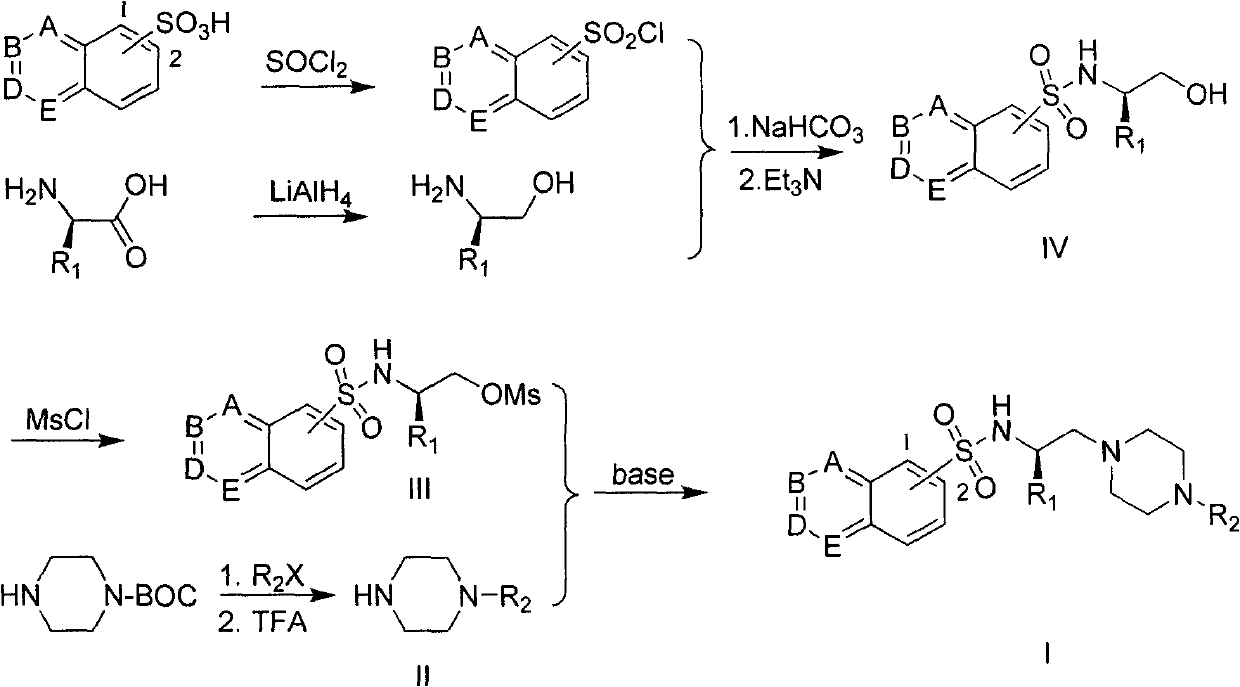
[0040] Example 1 : Preparation of N-[(1R)-2-hydroxyl-1-methylethyl]isoquinoline-5-sulfonamide (IV)
[0041]
[0042] This example relates to a general synthetic method of a class of substituted piperazine ethyl sulfonamide derivatives intermediate (IV). It specifically relates to the synthesis of N-[(1R)-2-hydroxyl-1-methylethyl]isoquinoline-5-sulfonamide. The compound is formed by condensation of L-propanol and isoquinoline-5-sulfonyl chloride.
[0043]Slowly add L-alanine (4.85g, 55.0mmol) to the tetrahydrofuran reaction solution (150mL) of lithium aluminum hydride (3.0g, 78.9mmol) under ice-cooling, add water and 15% NaOH solution after reacting for 20 hours The reaction was terminated, filtered, and the filtrate was evaporated to remove the solvent under reduced pressure to obtain a light yellow solid. The crude product was subjected to silica gel column chromatography to obtain 3.41 g of L-propanol as a colorless oil, with a yield of 82.5%. 1 H-NMR (CDCl 3 ): δ3.4...
Embodiment 2
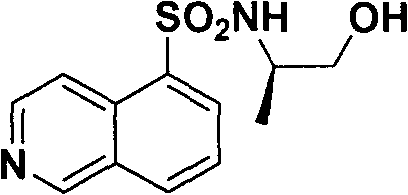
[0045] Example 2 : Preparation of (2R)-2-[(isoquinolin-5-ylsulfonyl)amino]propyl methanesulfonate (III)
[0046]
[0047] This example relates to a general synthetic method of a class of substituted piperazine ethylsulfonamide derivative intermediate (III). It specifically relates to the synthesis of (2R)-2-[(isoquinolin-5-ylsulfonyl)amino]propyl methanesulfonate. N-[(1R)-2-Hydroxy-1-methylethyl]isoquinoline-5-sulfonamide (3g, 13.0mmol) and triethylamine (2.01g, 19.9mmol) were dissolved in dry dichloromethane (120mL), after cooling in an ice bath, methanesulfonyl chloride (1.67g, 15.0mmol) was slowly added dropwise, washed with saturated sodium carbonate after half an hour, dried over anhydrous sodium sulfate, and the solvent was recovered under reduced pressure to obtain the crude product, the crude product Through silica gel column chromatography, 3.39 g of (2R)-2-[(isoquinolin-5-ylsulfonyl)amino]propyl methanesulfonate was obtained as a white solid, with a yield of 75...
Embodiment 3
[0048] Example 3 : Preparation of 1-(4-chlorobenzyl)piperazine (II)
[0049]
[0050] This example relates to a general synthetic method of a class of substituted piperazine ethyl sulfonamide derivatives intermediate (II). In particular it relates to the synthesis of 1-(4-chlorobenzyl)piperazine. Tert-butylpiperazine-1-carbonate (2.34g, 12.6mmol), 4-chlorobenzyl chloride (1.69g, 10.5mmol), triethylamine (2.1g, 20.5mmol) in acetonitrile (40mL) solution in After stirring at 60°C for 12 hours, the solvent was evaporated under reduced pressure, and the residue was partitioned and extracted with chloroform-water. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a crude product. The resulting crude tert-butyl-4-(4-chlorobenzyl)piperazine-1-carbonate (2.1 g, 0.01 mol) was dissolved in dichloromethane (4 mL), and trifluoroacetic acid (5.80 g, 0.08 mol), the reaction was contin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com