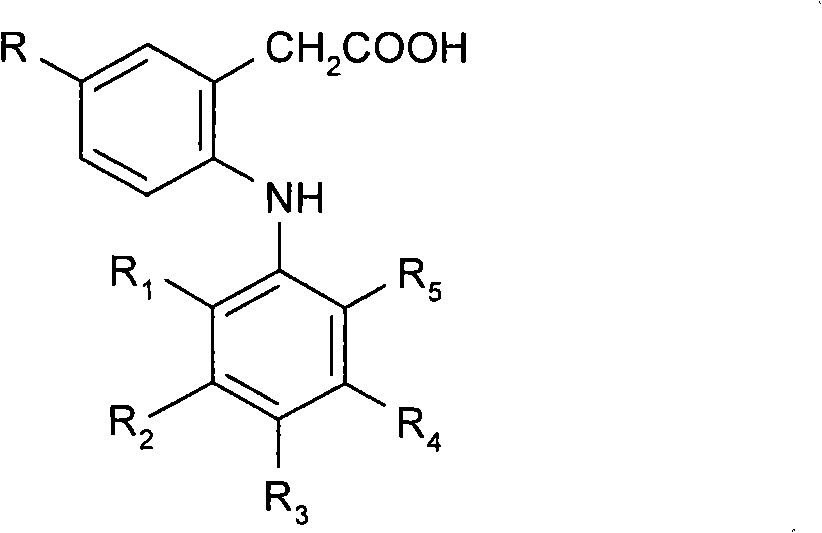
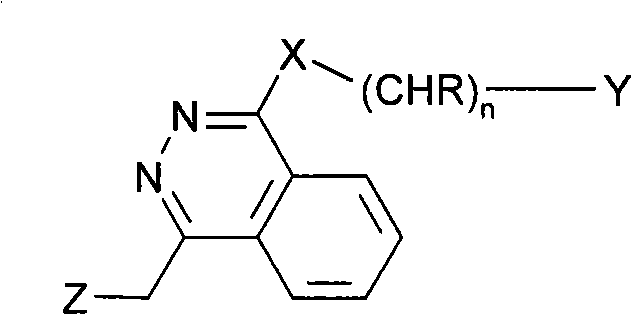
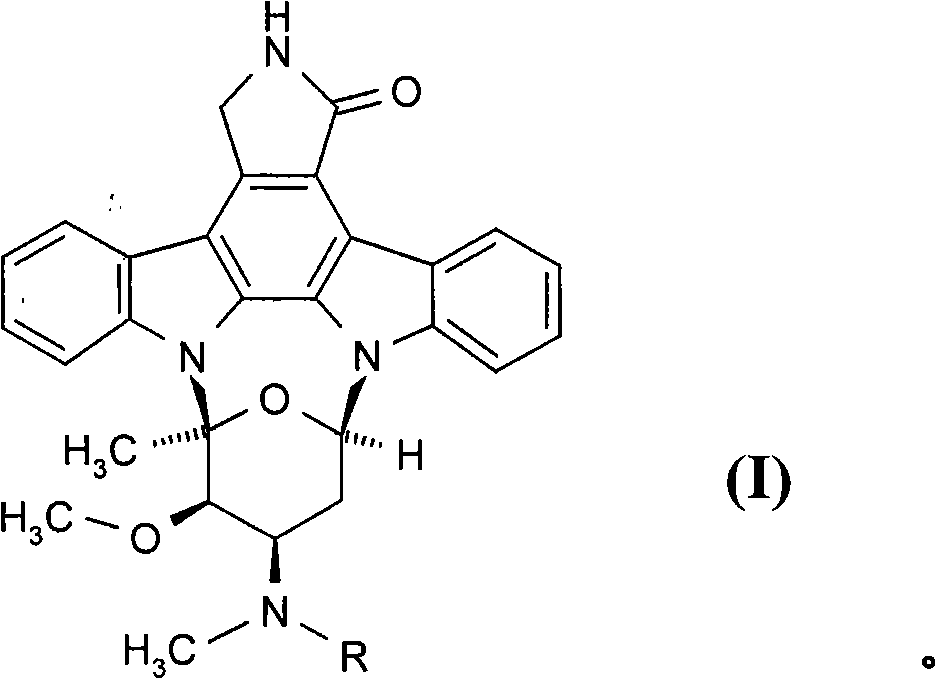
Ophtalmic ointment composition comprising a drug, an ointment base and a solubilizing/dispersing agent
A kind of technology of ophthalmic composition and ointment base, applied in the field of ointment, can solve problems such as being unsuitable for application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] A typical preservative-free ointment of the present invention is as follows:
[0147] Element
Example 1 1)
CGP 41251 2)
1.00
lanolin
5.52
White Vaseline
60.52
liquid paraffin
26.96
PEG400 3)
6.00
total
100
[0148] 1) Amount expressed in weight / weight percent (% w / w)
[0149] 2) The staurosporine derivative of formula (I), wherein R is benzoyl
[0150] 3) H-(OCH 2 -CH 2 ) n OH poly(ethylene glycol) wherein the index n has a value from about 8.5 to about 9 (average relative molecular weight of about 400).
[0151] Prepare as follows:
[0152] The staurosporine derivative is dissolved in poly(ethylene glycol) under heating and sonication, and the solution is thoroughly mixed with a molten ointment base containing lanolin, white petrolatum, and liquid paraffin. The resulting liquid composition is then stirred until room temperature is reached.
Embodiment 2 to 7
[0153] Embodiment 2 to 7 (ointment containing preservative)
[0154]
[0155] * Phenylethyl alcohol can be conveniently replaced, for example, by 0.01% benzalkonium chloride or cetrimonium bromide.
[0156] Preparation method
[0157] 1. Weigh the liquid paraffin in a media bottle. Remove air bubbles from the liquid paraffin by connecting to a vacuum for 30 minutes or until all air bubbles are removed.
[0158] 2. Melt white petrolatum and lanolin on a stirrer at about 70°C.
[0159] 3. Gently mix the liquid paraffin, white petrolatum, and lanolin at about 70°C for 15 minutes or until the ingredients are well mixed to form Part 1 .
[0160] 4. Dissolve PKC 412 in PEG400 or PEG 4000 by sonicating PKC 412 in a 45°C water bath. It takes about 4 hours to completely dissolve PKC 412. This is part 2.
[0161] 5. Add alpha-tocopherol and phenylethyl alcohol or benzalkonium chloride or cetrimonium bromide to PEG with PKC 412 (part 2). This is part 3.
[0162] 6. Add diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com