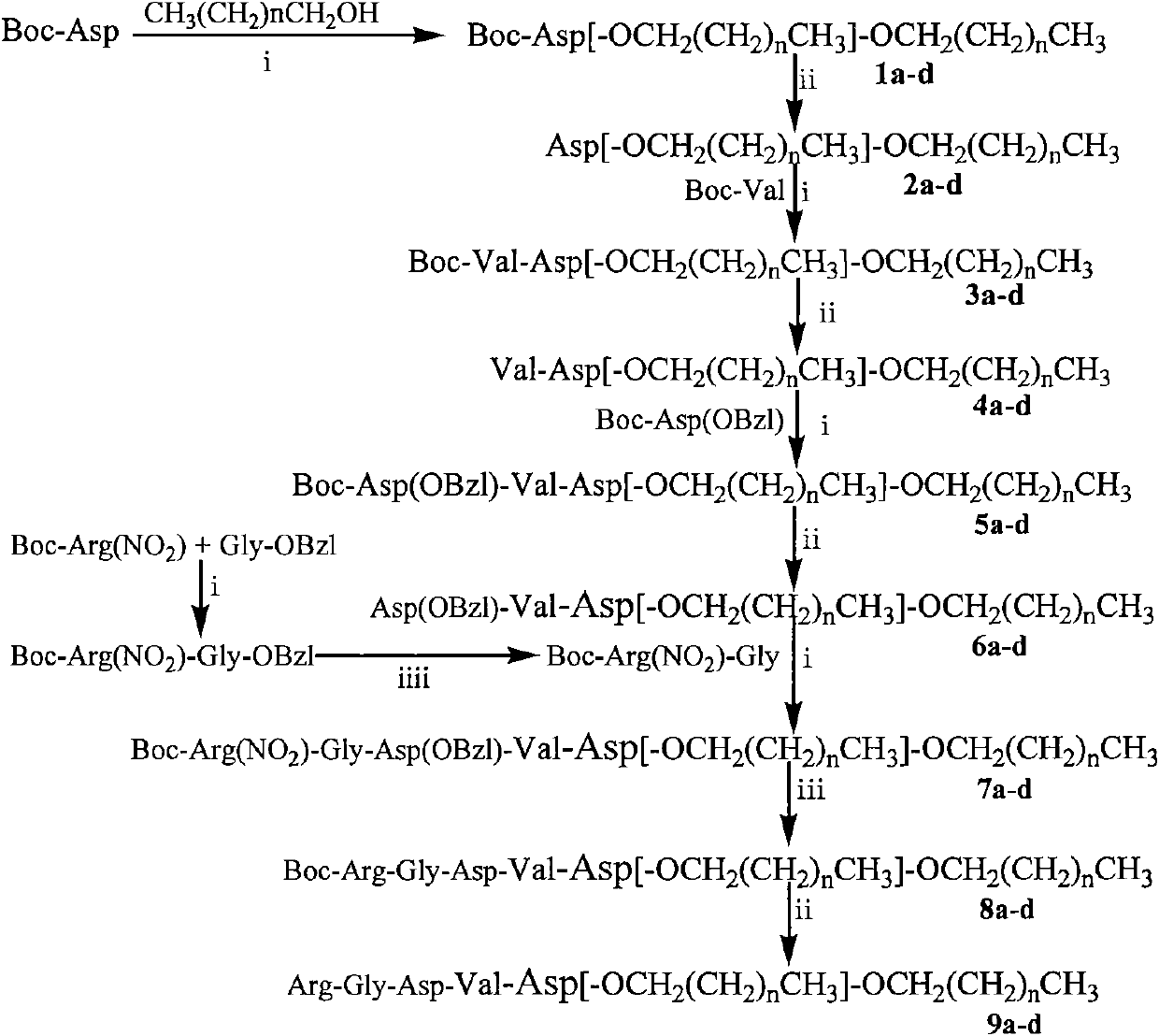
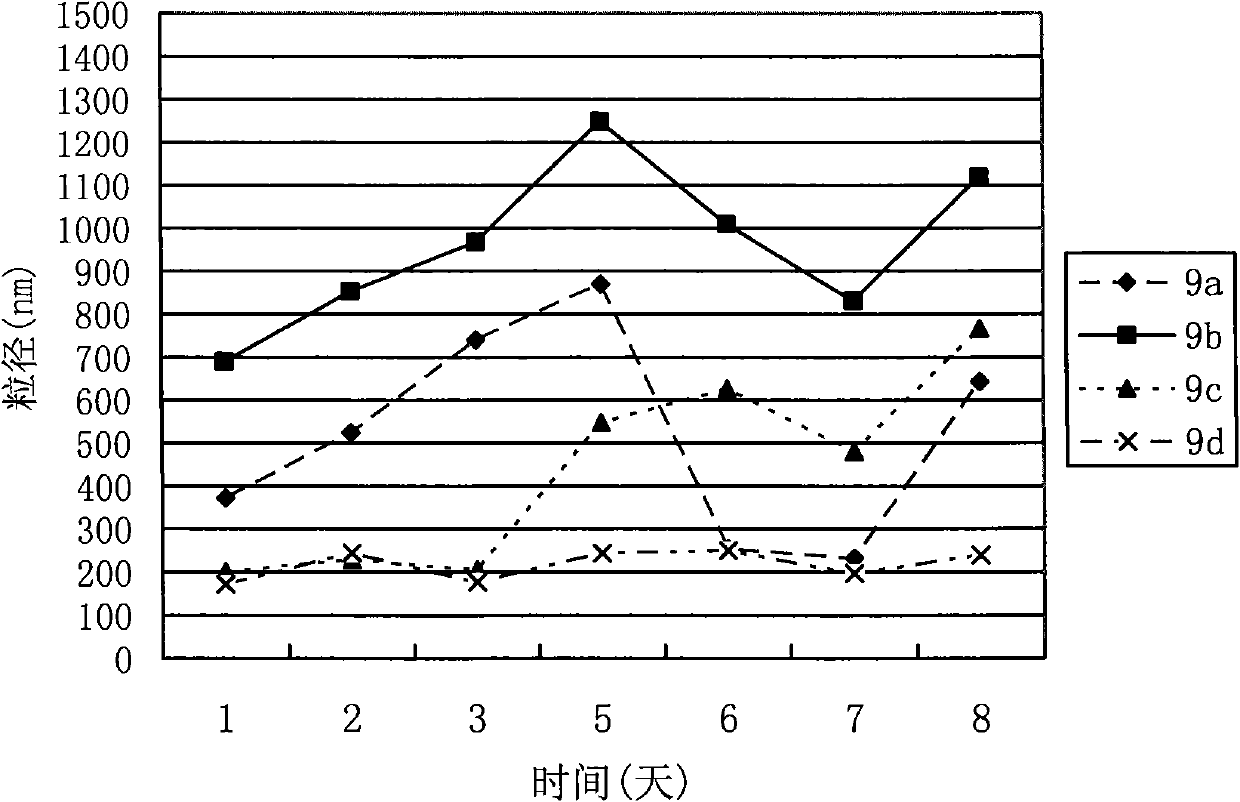
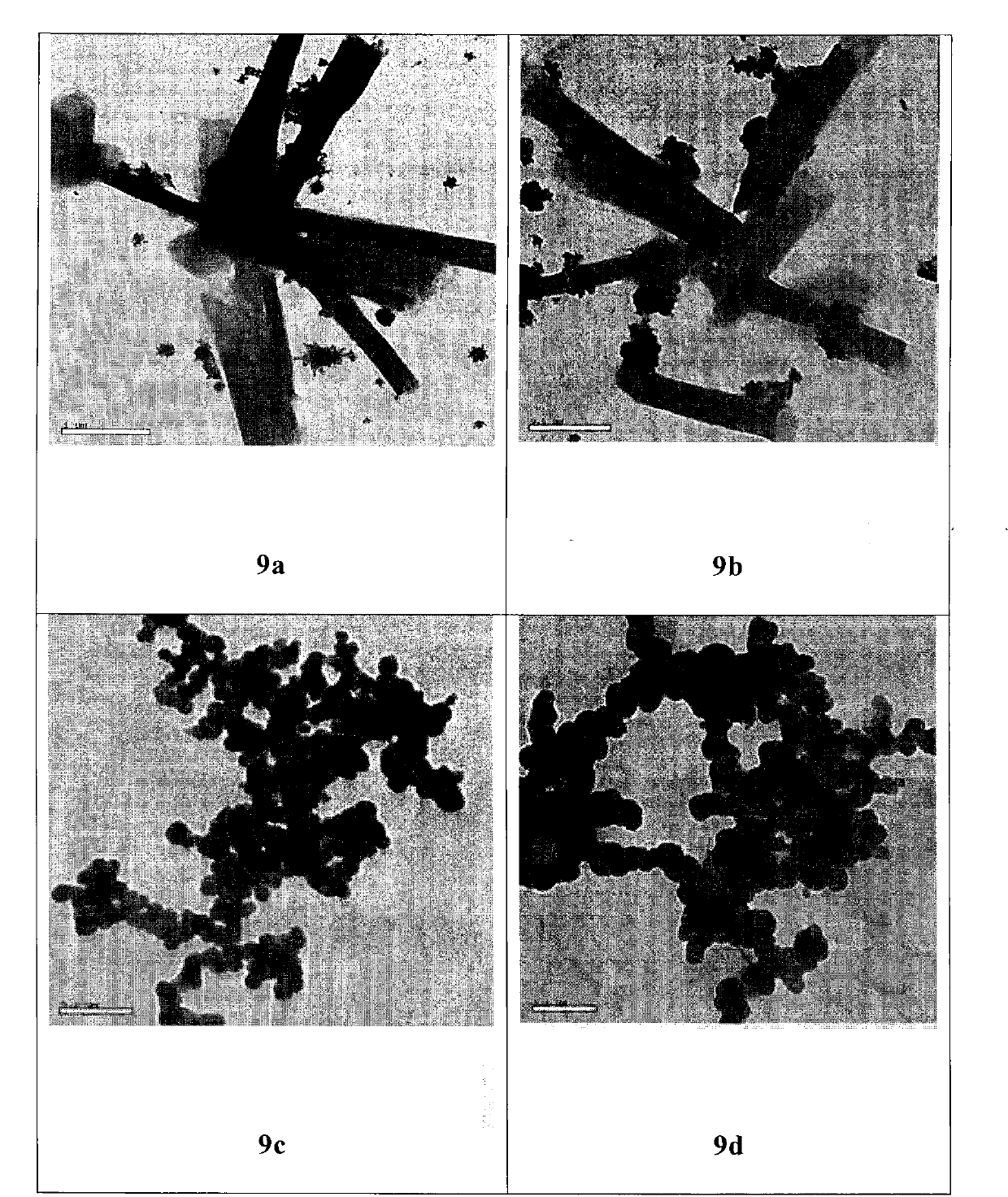
Conjugate formed by conjugating one Arg-Gly-Asp-Val chain and two fatty alcohol chains by Asp, synthesis thereof and medical application thereof
A technology of arg-gly-asp-val-asp and conjugates, applied in the field of biomedicine, can solve the problems of short half-life, less toxic and side effects, and low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0040] Embodiment 1Arg-Gly-Asp-Val-Asp[OCH 2 (CH 2 ) 6 CH 3 ] 2 Preparation of (9a)
[0041] 1) Boc-Arg (NO 2 )-Gly-OBzl:
[0042] 1.600g (5.0mmol) Boc-Arg (NO 2 ) was dissolved in 20ml of anhydrous THF to form a white emulsion, 0.745g (5.5mmol) of N-hydroxybenzotriazole (HOBt) was added under ice cooling, and 1.23g (6mmol) of dicyclohexylcarbonyldioxide was added after 10 minutes Amine (DCC), to obtain the reaction solution A, stand-by. Suspend 1.61g (5.25mmol) TOS H·Gly-OBzl in 20ml anhydrous THF under ice-cooling, then add N-methylmorpholine (NMM) to adjust the pH to 8-9. Stir for 35 minutes to obtain reaction solution B, which is ready for use. Add reaction solution A to reaction solution B under ice bath, first stir under ice bath for 1 h, then stir at room temperature for 12 h, TLC (chloroform / methanol, 10:1) showed that Boc-Arg (NO 2 )disappear. Dicyclohexylurea (DCU) was filtered off, and the filtrate was concentrated under reduced pressure to obtain a yello...
Embodiment 2A
[0063] Example 2Arg-Gly-Asp-Val-Asp[-OCH 2 (CH 2 ) 8 CH 3 ]-OCH 2 (CH 2 ) 8 CH 3 Preparation of (9b)
[0064] 1) Boc-Asp[-OCH 2 (CH 2 ) 8 CH 3 ]-OCH 2 (CH 2 ) 8 CH 3 (1b)
[0065] According to the preparation method of 1a, by 1.165g (5mmol) Boc-Asp and 1.659g (10.5mmol) CH 3 -(CH 2 ) 9 -OH yielded 2.565 g (99.9%) of the title compound as a colorless oil. ESI-MS (m / z): 536[M+Na] + , [ α ] D 20 = - 14.80 ( c = 1 , CH 3 OH ) .
[0066] 2) HCl·Asp[-OCH 2 (CH 2 ) 8 CH 3 ]-OCH 2 (CH 2 ) 8 CH 3 (2b)
[0067] Using the same method as in the preparation of 2a, 0.449 g (99.9%) of the target compound was prepared from 0.513 g (1 mmol) of 1 b as a colorless oil, which was directly...
Embodiment 3A
[0082] Example 3Arg-Gly-Asp-Val-Asp[-OCH 2 (CH 2 ) 10 CH 3 ]-OCH 2 (CH 2 ) 10 CH 3 Preparation of (9c)
[0083]1) Boc-Asp[-OCH 2 (CH 2 ) 10 CH 3 ]-OCH 2 (CH 2 ) 10 CH 3 (1c)
[0084] According to the preparation method of 1a, by 1.165g (5mmol) Boc-Asp and 1.953g (10.5mmol) CH 3 -(CH 2 ) 10 CH 2 -OH yielded 2.854 g (99.9%) of the title compound as a colorless oil. ESI-MS (m / z): 593[M+Na] + , [ α ] D 20 = - 8.93 ( c = 1 , CH 3 OH ) .
[0085] 2) HCl·H-Asp[-OCH 2 (CH 2 ) 10 CH 3 ]-OCH 2 (CH 2 ) 10 CH 3 (2c)
[0086] Using the same method as in the preparation of 2a, 0.506 g (99.9%) of the target compound was obtained from 0.569 g (1 mmol) of 1c as a colorless oil, which ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com