Modified leptin polypeptides and their uses
一种瘦素、修饰基的技术,应用在瘦素多肽领域,能够解决不具特异性、活性丧失、缺乏再现性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0214] I. Introduction
[0215] The present invention provides leptin molecules comprising at least one unnatural amino acid. In certain embodiments of the invention, the leptin polypeptide having at least one unnatural amino acid includes at least one post-translational modification. In one embodiment, said at least one post-translational modification comprises reacting a molecule comprising a second reactive group with at least one molecule comprising a first reactive group using chemistry known to those skilled in the art to be applicable to a particular reactive group. Molecules containing a second reactive group include (but are not limited to): labels, dyes, polymers, water-soluble polymers, derivatives of polyethylene glycol, photocrosslinking agents, cytotoxic compounds, drugs, affinity tags, photoaffinity tags, reactive compounds, resins, second proteins or polypeptides or polypeptide analogs, antibodies or antibody fragments, metal chelators, cofactors, fatty acids,...
example
[0717] The following examples are provided to illustrate, but not limit, the claimed invention.
example 1
[0719] This example describes one of many possible sets of criteria for selecting preferred sites in hGH for incorporation of non-naturally encoded amino acids.
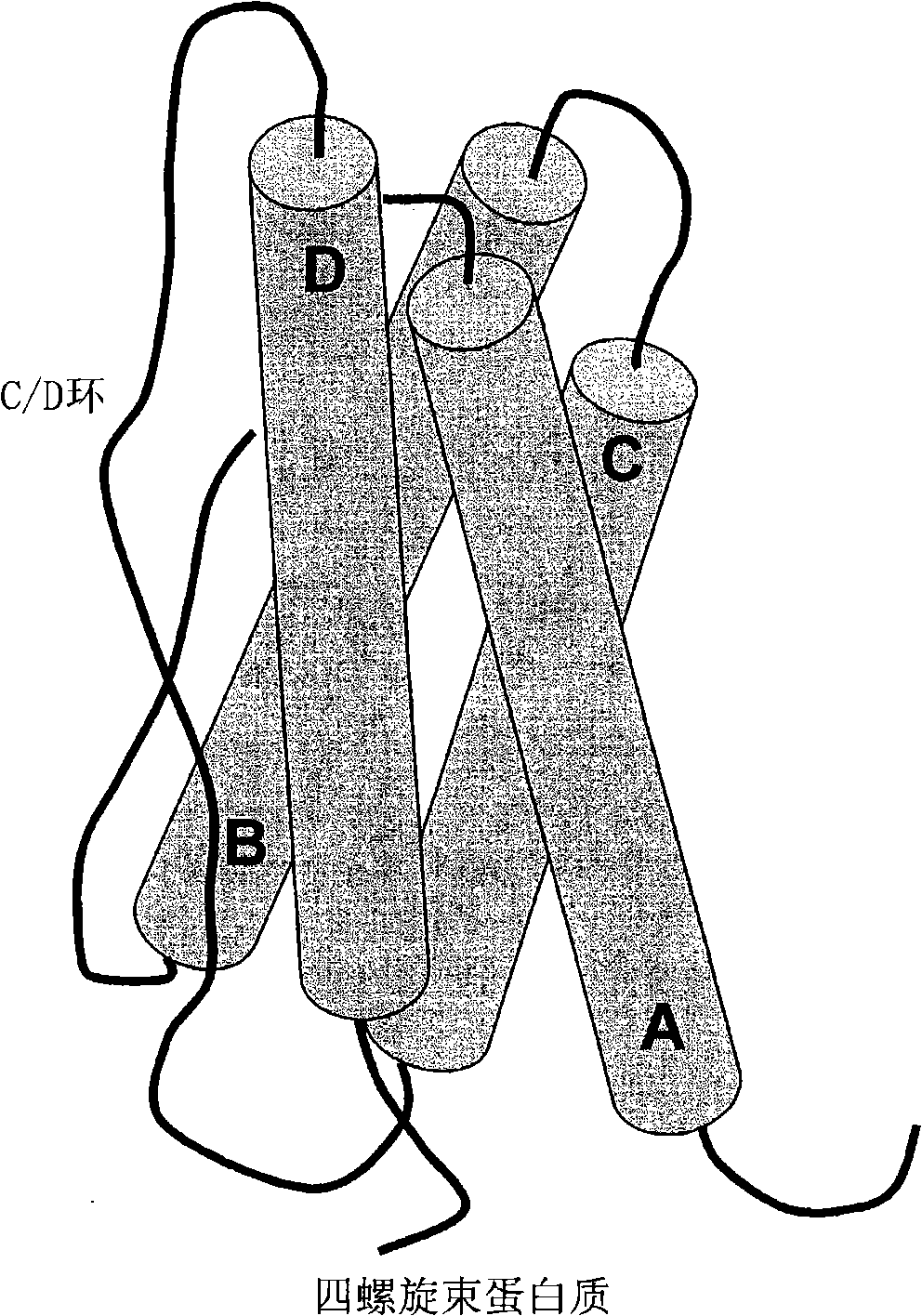
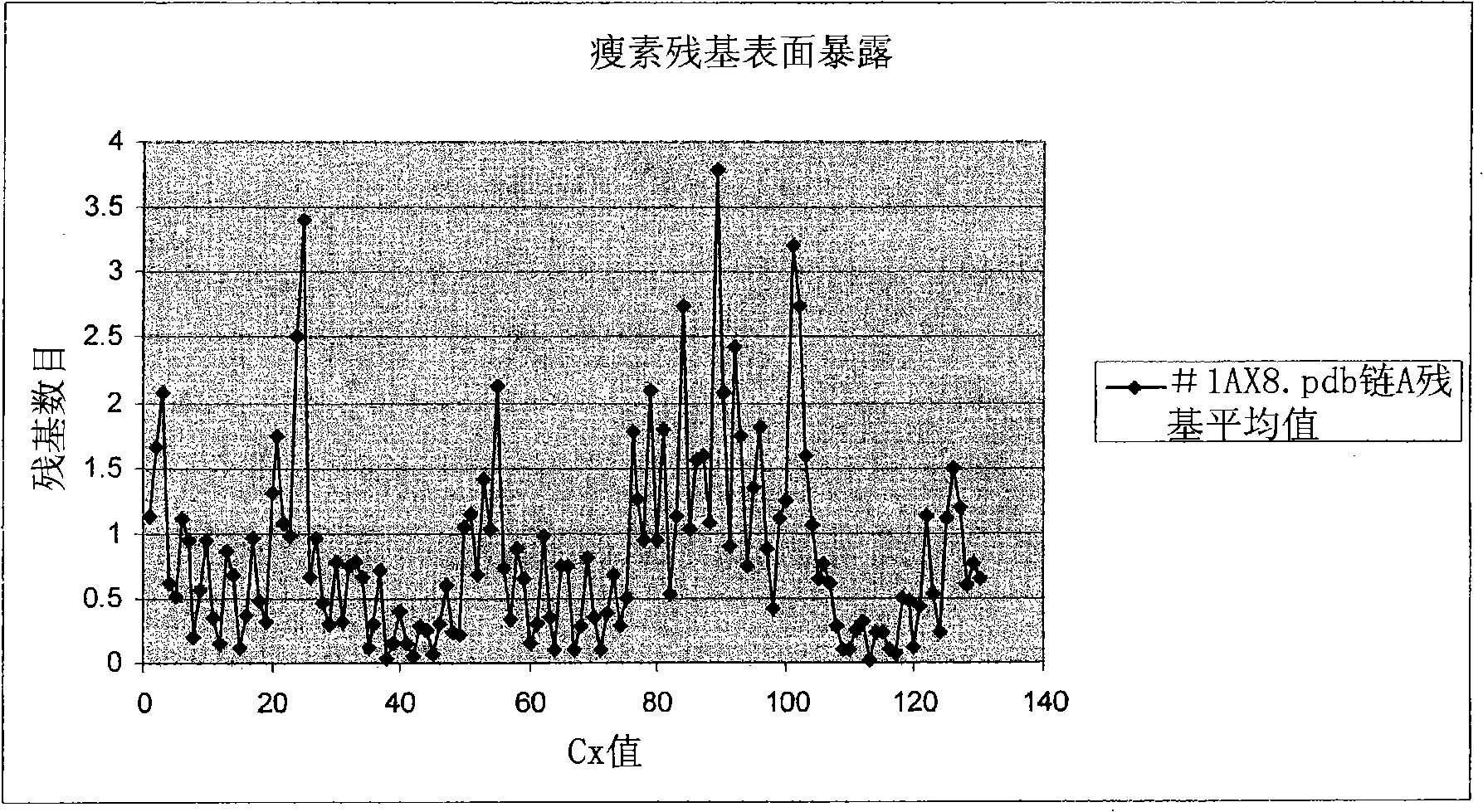
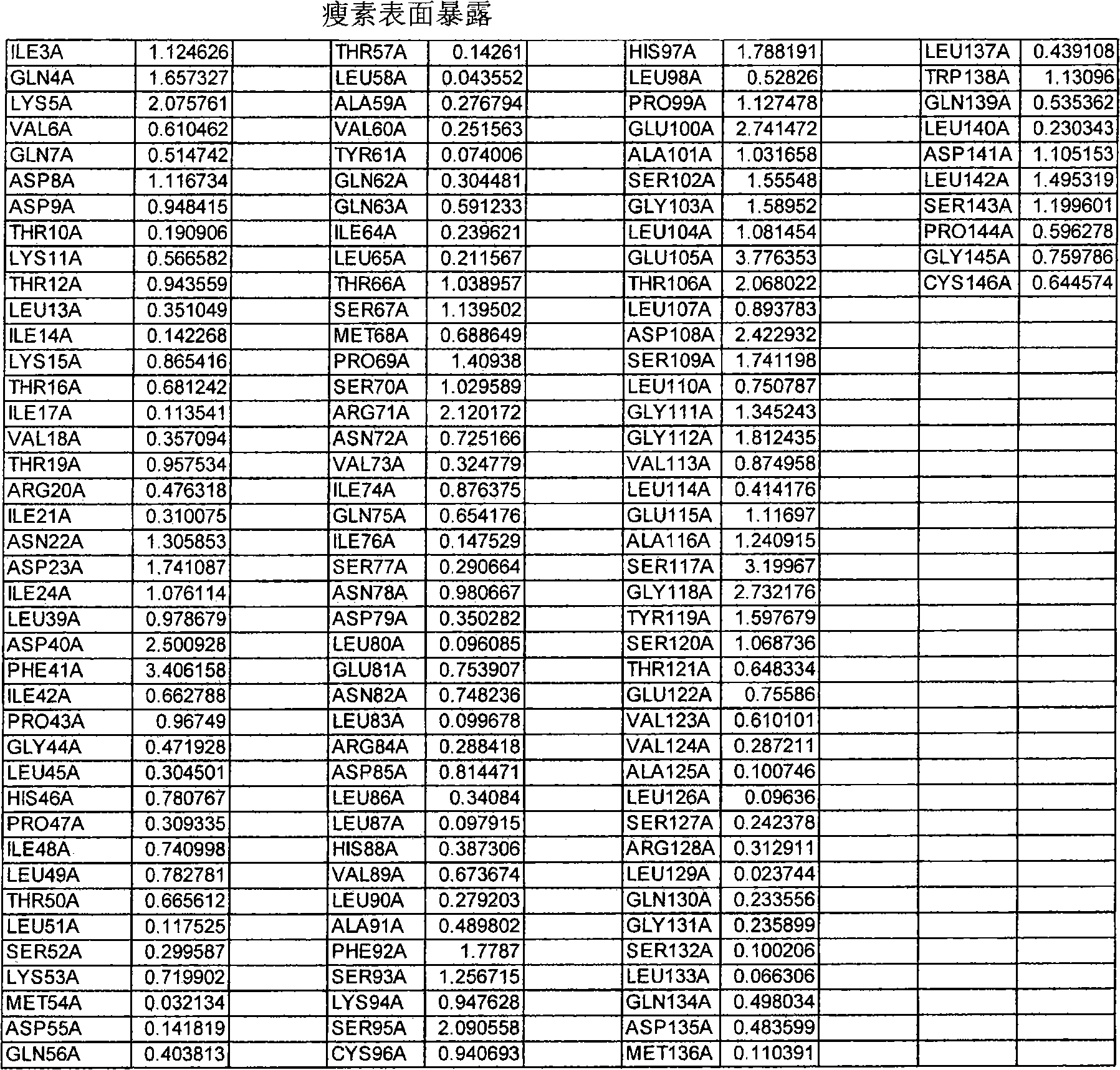
[0720] This example illustrates how to select preferred sites within hGH polypeptides for the introduction of non-naturally encoded amino acids. The crystal structure 3HHR consisting of hGH in complex with two molecules of the extracellular domain of the receptor (hGHbp) was used to determine preferred locations where one or more non-naturally encoded amino acids may be introduced. Other hGH structures (eg 1AXI) were used to examine possible variations in primary and secondary structural elements between crystal structure datasets. Coordinates for these structures are available from the Protein Data Bank (PDB) (Bernstein et al., J. Mol. Biol. 1997, 112, p. 535) or via the Structural Bioinformatics Research Consortium, available on the World Wide Web at rcsb.org Laboratory PDB (The Research Collaboratory for Structur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com