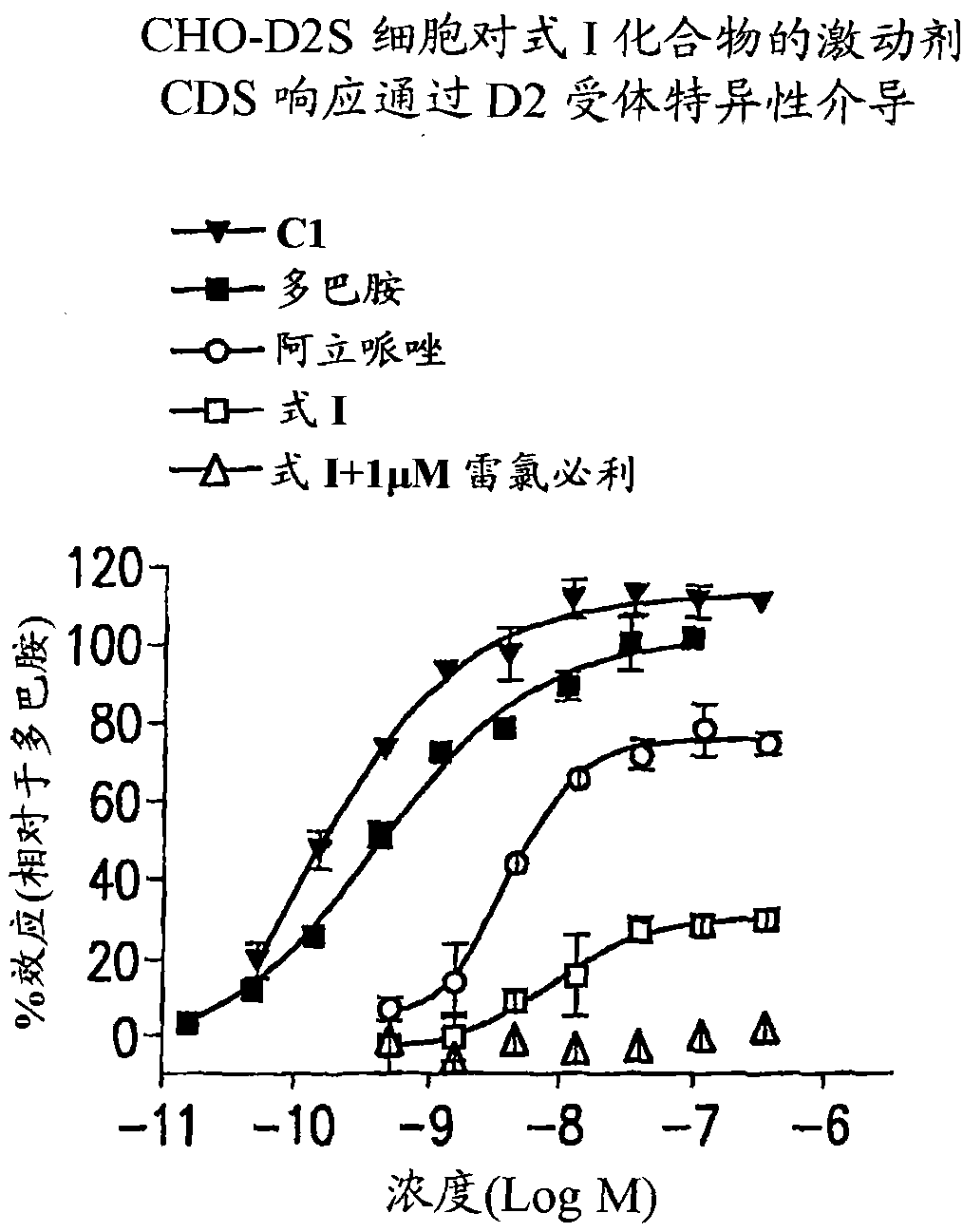
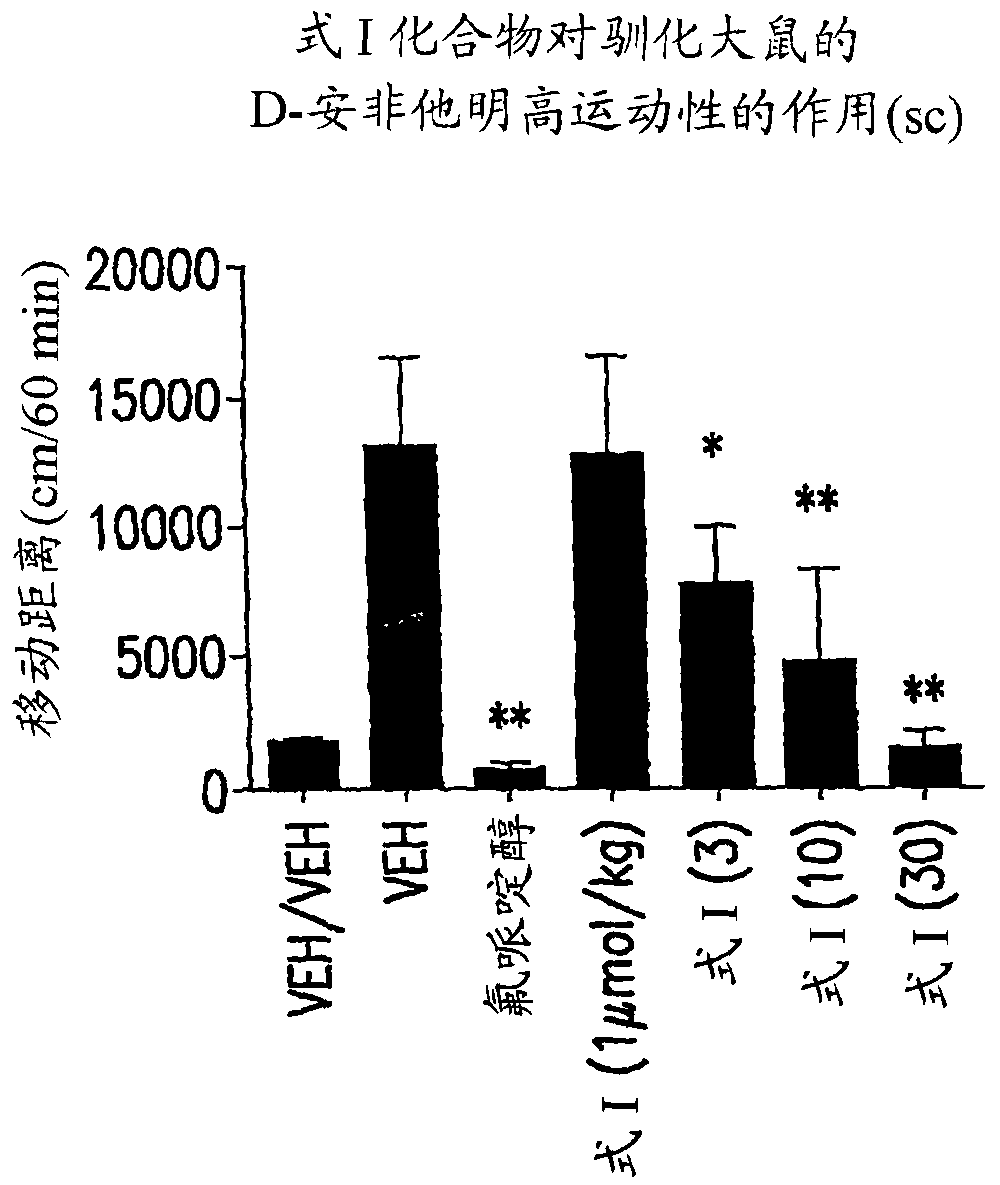
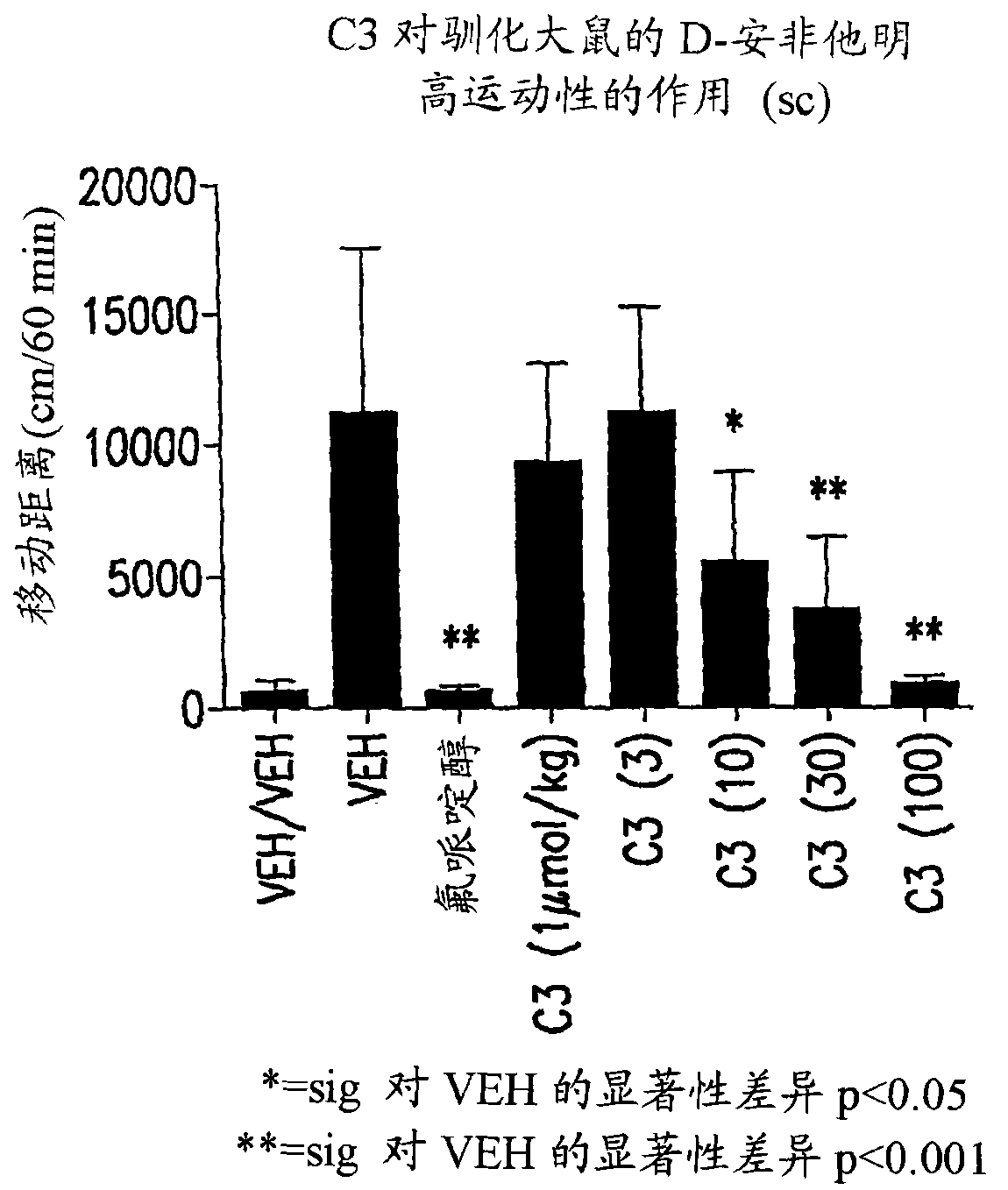
Compound (R) -N*6*-ethyl-6, 7-dihydro-5H-indeno (5, 6- d) thiazole-2, 6-diamine and the use as antipsychotics
A compound, 6-d technology, applied in nervous system diseases, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as limitations and lower patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1. (R)-N 6 -Synthesis of ethyl-6,7-dihydro-5H-indeno[5,6-d]thiazole-2,6-diamine
[0081] Scheme A below illustrates the synthesis of (R)-N 6 - The method of ethyl-6,7-dihydro-5H-indeno[5,6-d]thiazole-2,6-diamine.
[0082] Option A
[0083]
[0084] The synthesis is carried out as follows:
[0085] Step A. Synthesis of ((S)-5-bromo-indan-2-yl)-benzyl carbamate
[0086]
[0087] To (S)-5-bromo-indan-2-ylamine (1R)-(-)-10-camsylate (109.6 g, 246.8 mmol) cooled in an ice bath, as obtained by the Adv. Synth.Catal.2001, 343, prepared by the method given in pp 461-472) in CH 2 Cl 2 To the suspension in (1 L) was added DIEA (10 mL, 617 mmol) followed by benzyl chloroformate (36.5 mL, 259 mmol) dropwise over 5-10 minutes. The mixture was stirred for 2 h, during which time H was added 2 O (100 mL). The phases were separated and the organic phase was washed with 1M HCl (about 100 mL), H 2 O (about 100 mL) and saturated NaHCO 3 Aqueous solution (about 100mL...
Embodiment 2
[0116] Example 2. (R)-N 6 -Scale-up synthesis of ethyl-6,7-dihydro-5H-indeno[5,6-d]thiazole-2,6-diamine
[0117] Reaction Scheme A is scaled up approximately tenfold. Scale-up reactions were performed in a manner similar to that performed in Reaction Scheme A, except for the scale and the following differences:
[0118] In a scaled-up version of the method in Step A, the solvent consists of CH 2 Cl 2 into acetonitrile. This allowed product isolation by addition of water and subsequent filtration of the product. This scale-up method benefits from the absence of an extractive workup and the absence of halogenated solvents.
[0119] In the scale-up process, it was determined that the distillation of benzophenoneimine in step C was not necessary. Therefore, the distillation process was omitted without compromising the imine formation.
[0120] In steps E and F of the scaled-up method, the crude hydrolyzate was dissolved in toluene instead of CH 2 Cl 2 dissolved in. The b...
Embodiment 3
[0123] Example 3. In vitro assay process
[0124] The affinity (Ki) for the D2 receptor was used in [ 3 H]-raclopride (raclopride) binding assay to measure. D2 antagonism (IC 50 ) was measured with a GTPγS assay. However, the GTPγS assay cannot detect the agonistic effect of aripiprazole in the present inventors or others (Jordon S, et.al., "Dopamine D2 receptor partial agonists display differential or contrasting characteristics in membrane and cell-based assays of dopamine D2 receptor signaling," Prog Neuropsychopharmacol Biol Psychiatry, 31(2): 348-56 (March 30, 2007); Epub October 27, 2006). This highlights the limitations of using the GTPγS assay to identify D2 partial agonists. Assays that measure more downstream levels of cellular signaling such as cAMP and extracellular impedance across cell layers tend to detect agonism more sensitively to provide reliable agonism of D2 partial agonists. Changes in extracellular impedance across cell layers can be measured by cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com