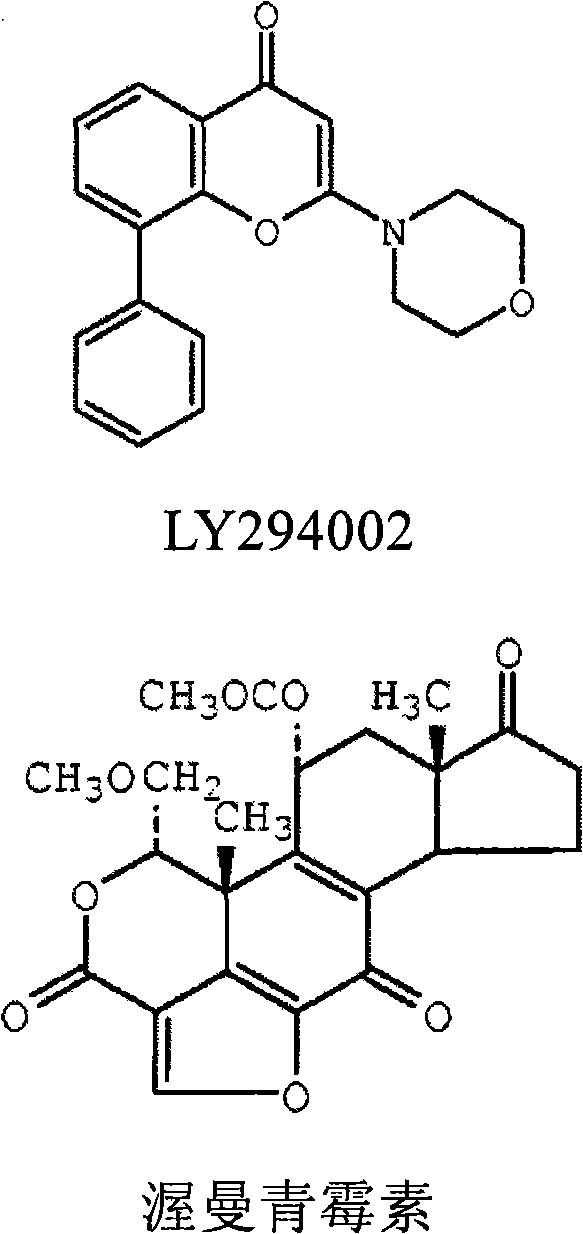
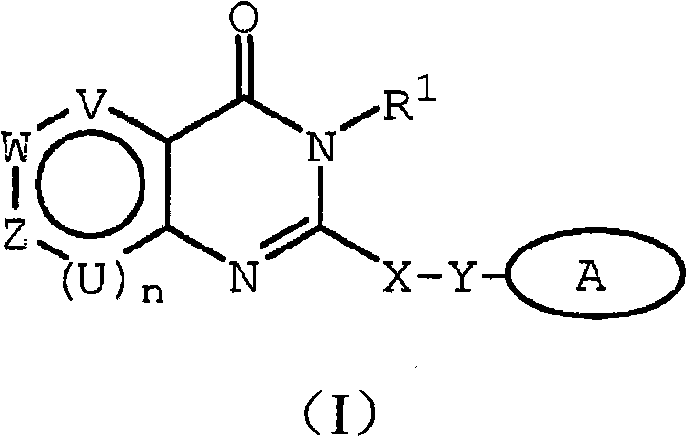
Inhibitors of human phosphatidyl-inositol 3-kinase delta
An alkylene, unsubstituted technology applied in the field of phosphatidylinositol 3-kinase, which can solve the problem of low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] Preparation and purification of recombinant PI3Kα, PI3Kβ and PI3Kδ
[0230] Use BAC-to-BAC The HT baculovirus expression system (GIBCO / BRL) overexpresses a recombinant PI3K heterodimer complex consisting of the p110 catalytic subunit and the p85 regulatory subunit, which is then purified for biochemical analysis. The four class I PI3-kinases were cloned into the following baculovirus vectors:
[0231] p110δ: Using standard recombinant DNA techniques, the FLAG -tagged human p110δ (SEQ ID NO: 1) (see Chantry et al., J Biol Chem, 272: 19236-41 (1997)) was subcloned into BamH1 of the insect cell expression vector pFastbac HTb (Life Technologies, Gaithersburg, MD) -Xba1 site, so that the clone is in frame with the His tag of the vector. The FLAG is described in US Patent Nos. 4,703,004, 4,782,137, 4,851,341 and 5,011,912 system, and reagents were obtained from Eastman Kodak Co.
[0232] p110α: Similar to the method used above for p110δ, the FLAG -tagged human p110α...
Embodiment 2
[0249] High Throughput Screening (HTS) and Selectivity Analysis of PI3Kδ
[0250] A high-throughput screen of a proprietary chemical library was performed to identify candidate inhibitors of PI3Kδ activity. PI3Kδ catalyzes phosphorylation from γ-[ 32 P]ATP is transferred to the PIP 2 PIP on the D3' site of the aliphatic ring of inositol 2 / PS liposomes. The reaction depends on MgCl 2 and ended in high molar potassium phosphate buffer, pH 8.0, containing 30 mM EDTA. In said screening, the reaction is performed in the presence or absence of library compounds. Reaction products (and all unlabeled products) were transferred to 96-well pre-wet PVDF (polyvinylidene (di)fluoride) filter plates, filtered, and washed in high molarity potassium phosphate. Scintillant was added to the dried wells and the overall radioactivity quantified.
[0251] Most analytical operations use BIOMEK A 1000 robotic workstation (Beckman) was performed and all plates were read using the Wallac liq...
Embodiment 3-7
[0260] Because PI3Kδ is expressed at significant levels in leukocytes, it is important to study the effects of selective inhibitors of PI3Kδ on leukocyte function. Accordingly, the effect of PI3Kδ inhibitors in several types of leukocytes was examined. Neutrophils were examined to determine the possible role of selective inhibition of PI3K[delta] (Example 3 below). It was surprisingly found that selective inhibition of PI3Kδ activity was significantly associated with inhibition of some, but not all, functions of activated neutrophils. In addition, the effects of PI3K[delta] inhibitors on B cell and T cell function were analyzed (Examples 4-5 below). Furthermore, since PI3Kδ is also expressed in osteoclasts, the effect of PI3Kδ inhibition on these specific cellular functions was also investigated (Example 6 below). The effect of PI3K[delta] on basophil function was also investigated (Example 7 below).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap