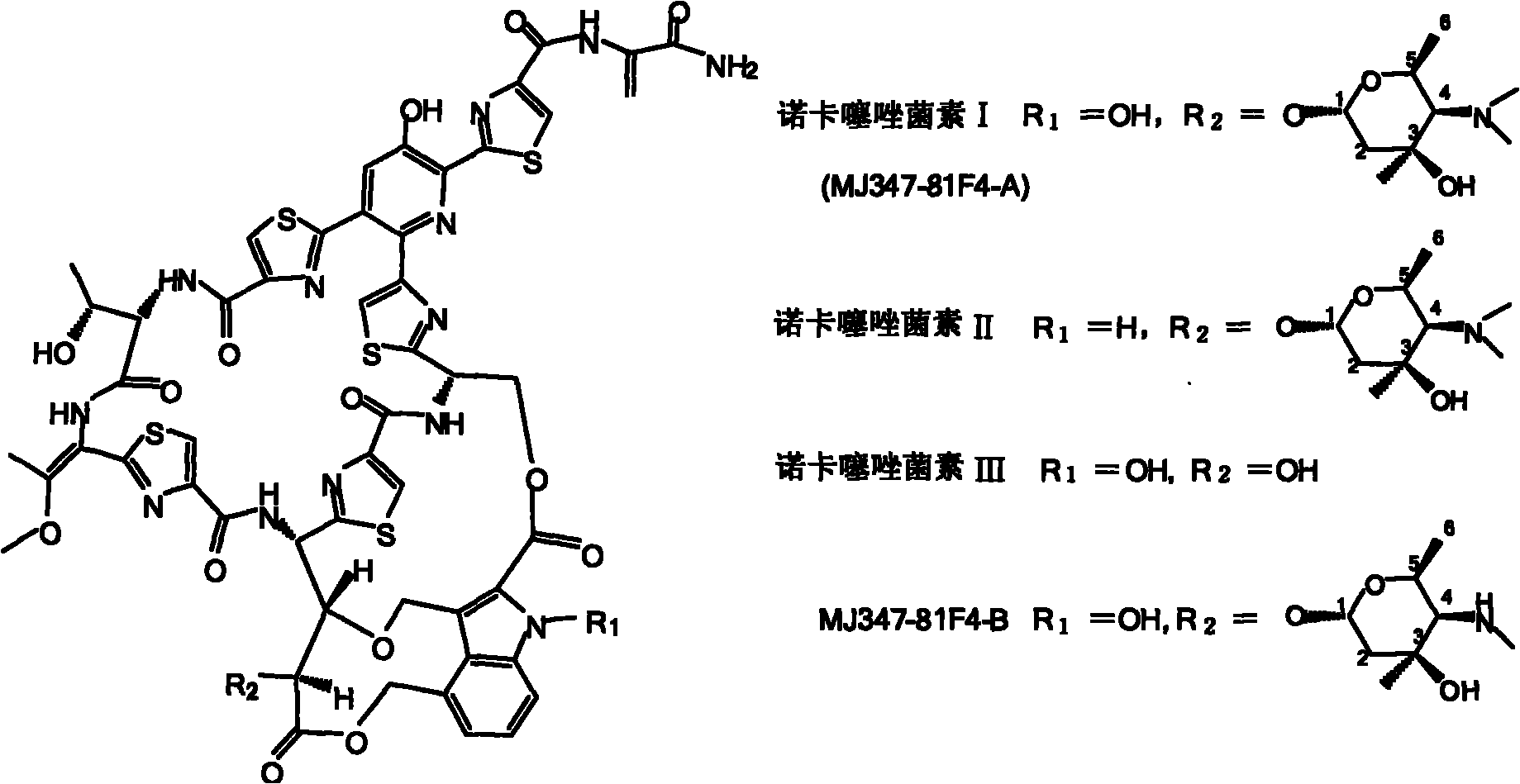
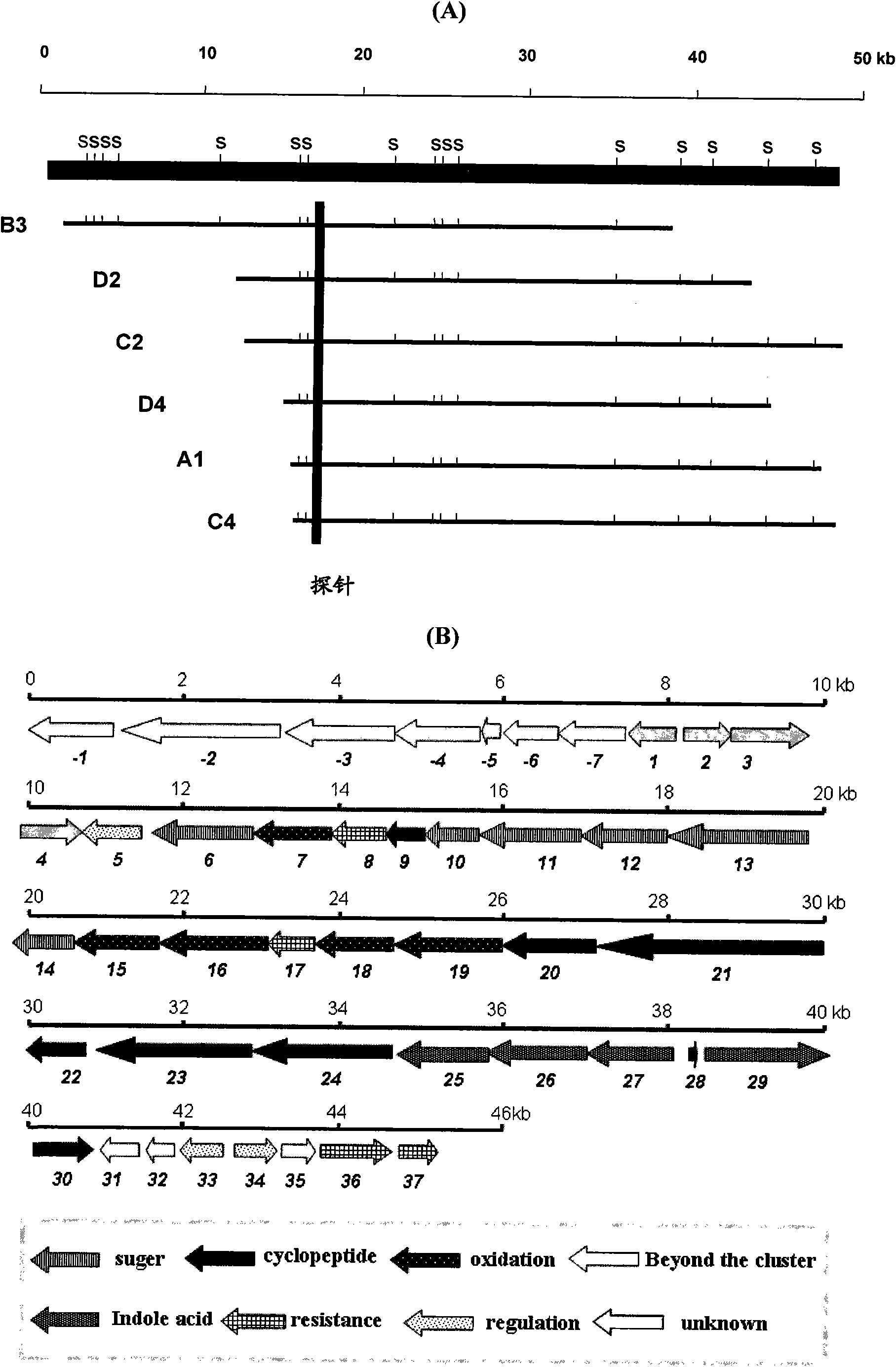
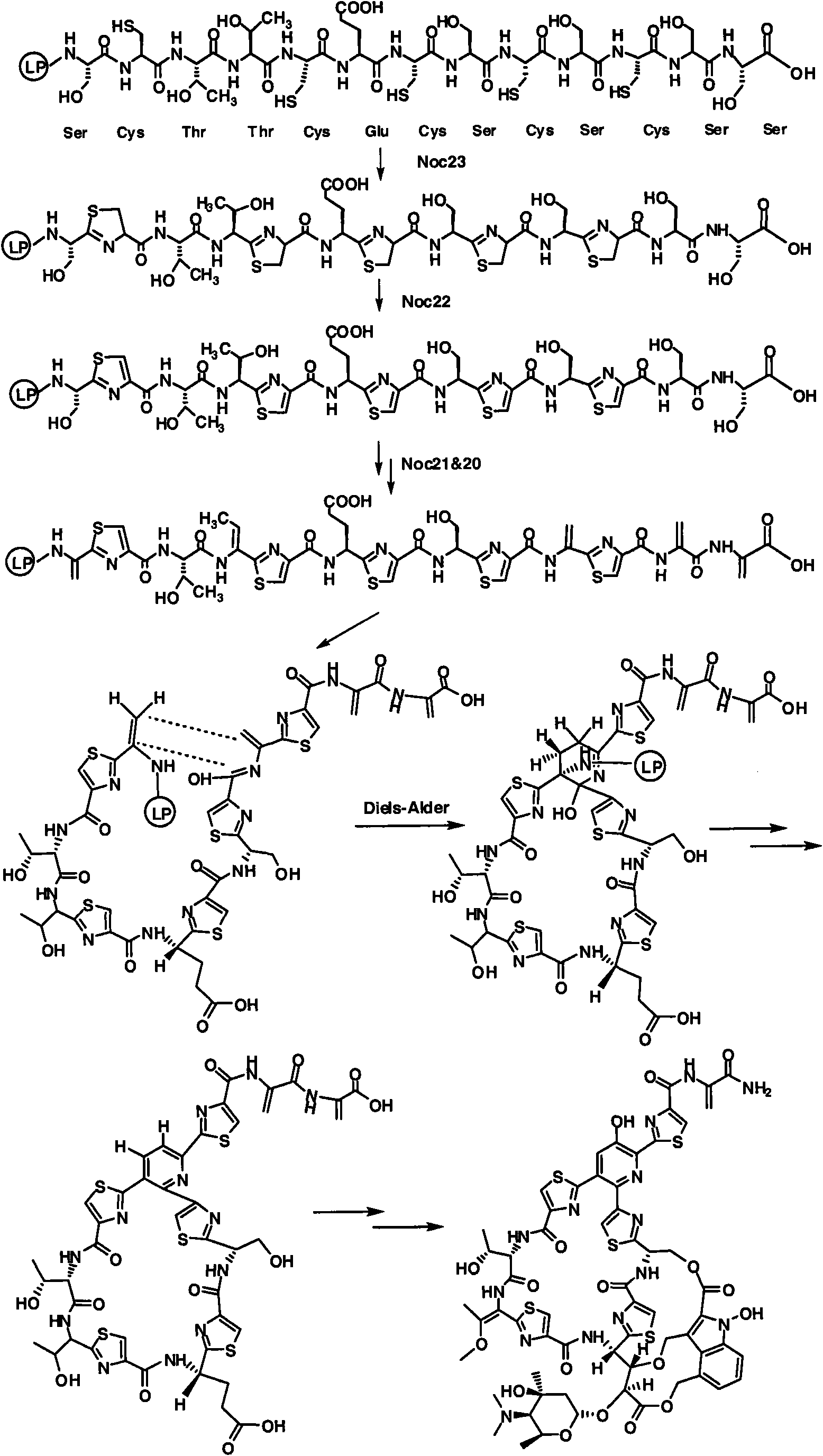
Biosynthesis gene cluster of Nocathiacins and application thereof
A technology for nocardiazol and biosynthesis, which is applied in the fields of microbial gene resources and genetic engineering, and can solve the problems of complex chemical structure, high production cost, numerous transformation steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Extraction of total DNA from Nocardia sp.WW-12651, a nocardiazolin-producing bacterium:
[0145] Inoculate 100 μL of Nocardia sp.WW-12651 (ATCC 202029) mycelia suspension into 3 mL of TSB liquid medium, culture at 30° C., 250 rpm, and shake for about 36 hrs to reach the late logarithmic growth phase. Take 3mL and inoculate into 50mL TSB (containing 5mM MgCl 2 , 0.5% glycine), 30 DEG C, 250rpm, shake culture for about 25hrs and reach the early stage of the stable growth period, it is milky white and turbid, and there are a large number of suspended mycelia. Centrifuge the bacterial solution at 4°C, 3500 rpm for 15 minutes to collect mycelia, wash twice with lysis buffer to obtain about 2.5 mL of mycelia. Add 10 mL of lysis buffer (containing 5 mg / mL of lysozyme) to 2.5 mL of mycelium, vortex until uniform, then add Achromopeptidase (Achromopeptidase) to 3 mg / ml, and mix well. 37°C water bath for 30min. Add 0.1mL proteinase K (20mg / mL, freshly prepared with lysis buffe...
Embodiment 2
[0147] Construction of Genome Library of Nocardia sp.WW-12651, a Nocardiazolin-producing Bacteria:
[0148] First, through a series of dilution experiments to determine the amount of Sau3AI, on this basis, a large number of enzyme-digested DNA fragments slightly larger than 40kb, dephosphorylated. The vector pOJ446 (Gene 1992, 116: 43-49; US 7,109,019) was first cut and dephosphorylated from the middle of the two cos sequences with HpaI, and then cut with BamHI from the multiple cloning site to obtain two tethered arms, and The prepared DNA fragments with a length of about 40kb were ligated overnight. Take out the packaging kit (Promega PackageneExtract) from -80°C and put it on ice. After it just melts, add 5uL of the above connection solution immediately, flick and mix well, be careful not to generate air bubbles, and place at room temperature (about 22°C) for 3 hours. Add 445uL Phage buffer and mix by inverting up and down. Then add 25uL of chloroform, mix well by inverti...
Embodiment 3
[0151] Fermentation, product isolation, purification and identification of Nocardia sp.WW-12651, a nocardiazolin-producing bacterium:
[0152] First, inoculate 300 μL of Nocardia sp.WW-12651 mycelial suspension frozen at -80°C into 25 mL of seed medium (soluble starch 2%, glucose 0.5%, N-Z Case 0.3%, yeast extract 0.2%, fish meat Extract 0.5%, calcium carbonate 0.3%), 32 ℃, 250rpm cultivated for 3 days. Get 2mL therefrom and transfer to 50mL fermentation medium (glucose 2%, HY-yeast 4121%, nutritional soybean 1%), 30 ℃, 250rpm culture 4-5 days. Then the fermentation broth was combined, transferred to a centrifuge tube, centrifuged at 3800rpm for 15min, and the mycelia precipitate was discarded. The supernatant was extracted twice with an equal volume of ethyl acetate, and the organic phases were combined. with anhydrous MgSO 4 or anhydrous Na 2 SO 4 Dry, filter, and concentrate to dryness under reduced pressure at 37°C. The sample was dissolved in a mixed solvent of chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com