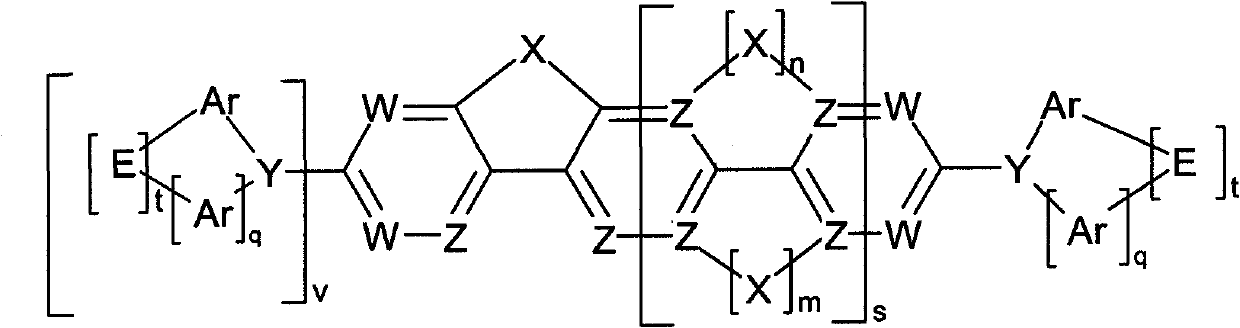
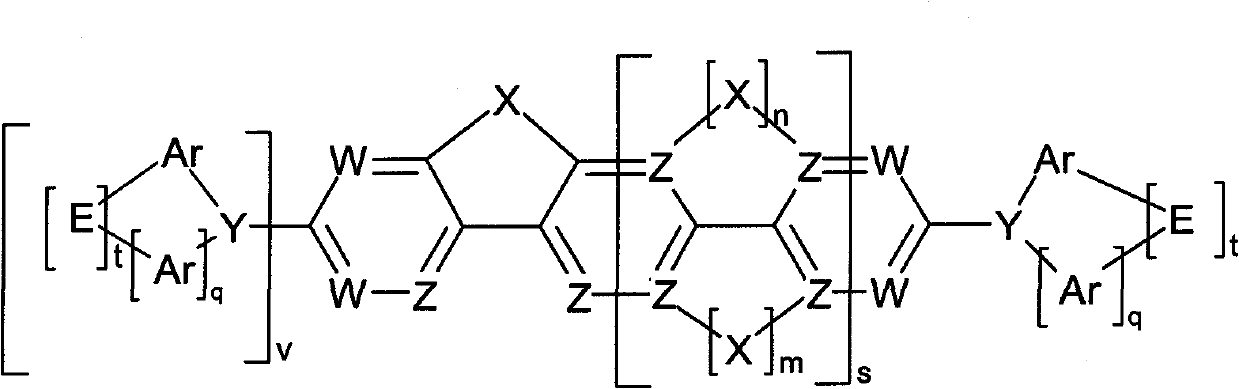
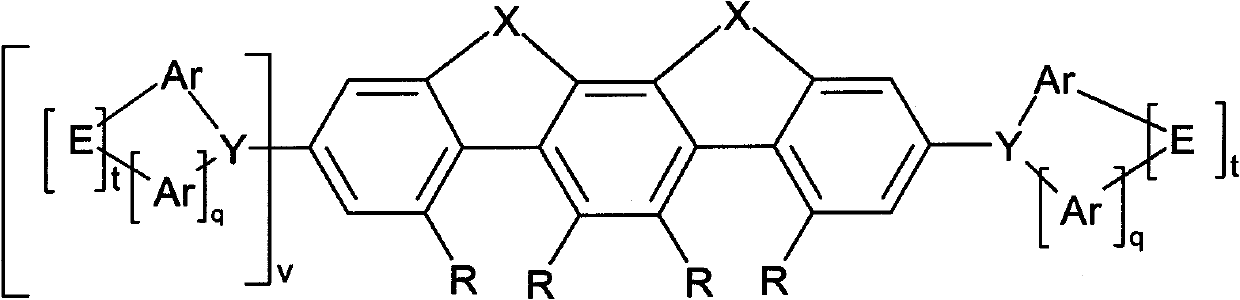
Compounds for electronic devices
A compound and atomic technology, applied in the field of compounds used in electronic devices, can solve problems such as high working voltage, blocking vapor deposition source, high working voltage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Example 1: 4,6,6,10,12,12-hexamethyl-N,N,N',N'-tetraphenyl-6H,12H-indeno[1,2-b]fluorene-2 , 8-diamine
[0150] a) Diethyl 2,2″-dimethyl[1,1′; 4′,1″]terphenyl-2′,5′-dicarboxylate
[0151]
[0152] 60g (160mmol) of dibromodiethyl terephthalate, 43g (320mmol) of o-tolylboronic acid, 365mg (0.32mmol) of Pd (PPh 3 ) 4 and 92g (660mmol) of K 2 CO 3 Heated in 300ml of toluene and 300ml of water for 4h under boiling. The mixture was then partitioned between toluene and water, and the organic phase was washed three times with water and washed with Na 2 SO 4 dry. The remaining residue was recrystallized twice from heptane to yield colorless crystals. Yield is 51 g (127 mmol, 81%).
[0153] b) 2-[5′-(1-hydroxyl-1-methylethyl)-2,2″-dimethyl[1,1′; 4′,1″]terphenyl-2′-yl]propane -2-ol
[0154]
[0155] 39 g (97 mmol) of the diester were initially introduced into 500 ml of dry THF, 195 ml (580 mmol) of a 3 M solution of methylmagnesium chloride in THF were added to the...
Embodiment 2
[0165] Example 2: 5,6,11,11,12,12-hexamethyl-N,N,N',N'-tetraphenyl-11H,12H-indeno[2,1-a]fluorene-2 , 9-diamine
[0166] a) 1,4-dibromo-2,3-xylene
[0167]
[0168] The compound was prepared according to Cachia, Wahl, Bull. Soc. Chim. Fr. 1958, 1418-1420.
[0169] b) 2,3-xylene-1,4-bisboronic acid pinacyl ester
[0170]
[0171] 100g (380mmol) of 1,4-dibromo-2,3-xylene was dissolved in 1500ml of dry ether, and 420ml (840mmol) of 2M n-butyllithium in cyclohexane was added dropwise at -70°C, After 1 h, 130 ml of trimethyl borate (1140 mmol) were added dropwise, the mixture was brought to RT during 1 h, the solvent was removed, 90 g (76 mmol) of pinacol and 1000 ml of toluene were added, and the mixture was heated at boiling for 2 h , the solvent is removed again, and the 1 H-NMR showed a homogeneous residue which was used in the subsequent reaction without further purification.
[0172] c) 2′,3′-dimethyl[1,1′;4′,1″]terphenyl-2,2″-dicarboxylic acid dimethyl ester
[01...
Embodiment 3
[0187] Example 3: 4,7,11,11,12,12-hexamethyl-N,N,N',N'-tetraphenyl-11H,12H-indeno[2,1-a]fluorene-2 , 9-diamine
[0188]
[0189] a) o-methylcinnamaldehyde
[0190]
[0191] This compound was synthesized according to Battistuzzi et al., Organic Letters 2003, 5(5), 777-780.
[0192] b) Diethyl 2-methylbenzylphosphonate
[0193]
[0194] The compound was synthesized according to de Meijere et al., Eur. J. Org. Chem. 1998, 2289-2299.
[0195] c) 2,2'-Dimethyltransstilbene
[0196]
[0197] The compound was synthesized according to de Meijere et al., Eur. J. Org. Chem. 1998, 2289-2299.
[0198] 5.99 g (149.9 mmol, 60% suspension in paraffin oil) of NaH were suspended in 250 ml of THF under argon atmosphere. The mixture was cooled to 0° C. and 37.28 g (153.9 mmol) of diethyl 2-methylbenzylphosphonate in 20 ml THF were added during 15 min. 19.74 g (135.0 mmol) o-cinnamaldehyde dissolved in 20 ml of THF were then added. The reaction was then stirred at RT for severa...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap