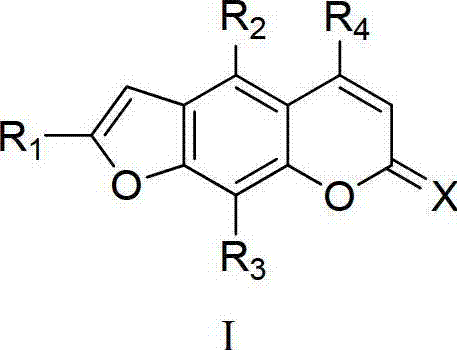
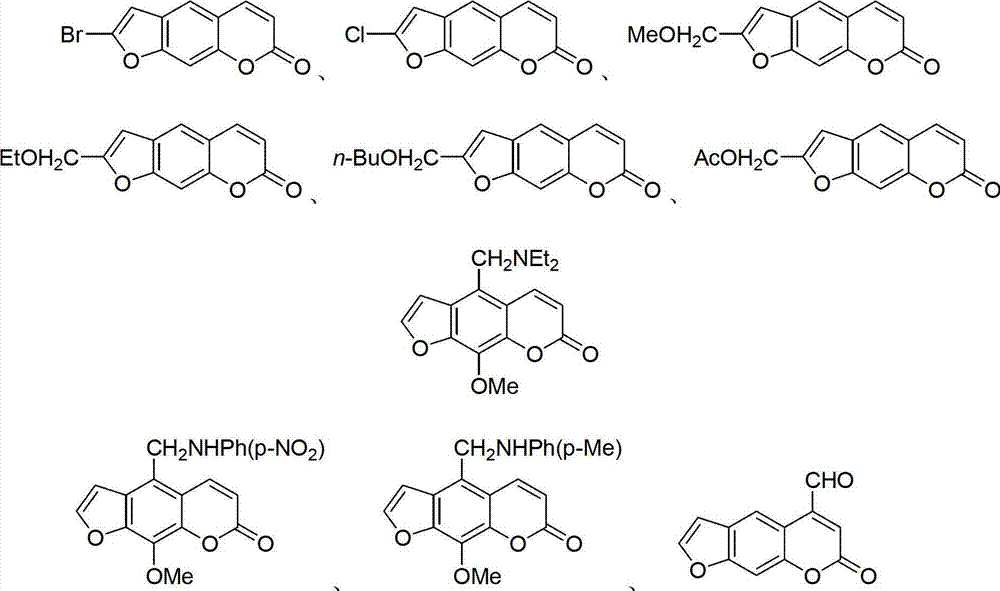
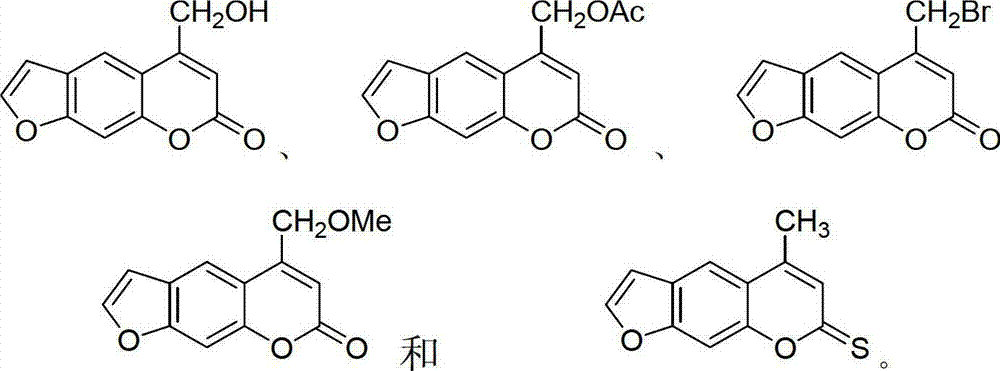
Furocoumarin compound and application thereof
A technology of furanocoumarins and compounds, applied in the field of pharmaceutical applications, can solve the problems of poor patient tolerance, large side effects, curative effect and application impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the synthesis of Zb-2a and Zb-2b
[0052]
[0053] Zb-2a R 1 =Br
[0054] Zb-2b R 1 = Cl
[0055] (i) NBS / NCS, CCl 4 , reflow, 9h
[0056] Into a 10 mL round bottom flask were added 21.6 mg of psoralen, 1.1 equivalent (eq.) of NBS, and 5 mL of carbon tetrachloride. The temperature was raised to reflux under stirring for 9h. After cooling, the solvent was evaporated under reduced pressure, and the crude product was subjected to flash silica gel column chromatography (petroleum ether: CH 2 Cl 2 =1:1) to obtain the target compound 2'-bromofuranocoumarin Zb-2a as a light yellow solid with a yield of 32%.
[0057] Except that NCS was used instead of NBS in the 2′-bromofuranocoumarin synthesis step, 2′-chlorofuranocoumarin Zb-2b was synthesized in the same procedure as an off-white solid with a yield of 25%.
Embodiment 2
[0058] Embodiment 2: the synthesis of compound Zb-4a~Zb-4c and Zb-4d
[0059]
[0060] Zb-4a: R=OMe,
[0061] Zb-4b: R=OEt,
[0062] Zb-4c: R=OBu-n,
[0063] Zb-4d: R=OAc,
[0064] (i) MOMCl, HOAc, room temperature, 36h; (ii) Zb-4a~Zb-4c: ROH (R=Me, Et, n-Bu), reflux, 3h;
[0065] Zb-4d: NaOAc, HOAc, (Ac) 2 O, 110°C, 3h;
[0066] Step 2.1: Add 300 mg psoralen and 10 mL glacial acetic acid to a 25 mL round bottom flask. After stirring evenly, 2 mL MOMCl was added to the above reaction system. Under stirring at room temperature, the reaction was sealed until the starting material disappeared (about 36h, detected by TLC). Add 20 mL of water, extract with EtOAc (30 mL×3), wash with saturated sodium bicarbonate, wash with water, and dry over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and the crude product was eluted by flash silica gel column chromatography gradient (petroleum ether: EtOAc=4:1~2:1) to obtain 221 mg of c...
Embodiment 3
[0070] Embodiment 3: the synthesis of compound Zb-11c~Zb-11g
[0071]
[0072] R=OPr -n ,OBu -n , NEt 2 ,
[0073] NHPh-p-NO 2 , NHPh-p-OMe
[0074] (i) MOMCl, HOAc, room temperature, 14h; (ii) Zb-11c~Zb-11d: ROH (R=n-Pr, n-Bu), reflux;
[0075] Zb-11e~Zb-11g: Et 2 NH (or p-NO 2 PhNH 2 , or p-MeOPhNH 2 ), K 2 CO 3 , acetone, reflux, 3h;
[0076] Step 3.1: Add 500 mg of 8-methoxypsoralen and 3 mL of glacial acetic acid to a 25 mL round bottom flask. After stirring evenly, add 1 mL MOMCl to the above reaction system. Under stirring at room temperature, the reaction was sealed until the starting material disappeared (about 14 h, detected by TLC). Add water 20mL, CH 2 Cl 2 Extract (30mL×3), wash with water, and dry over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and the crude product was eluted by flash silica gel column chromatography gradient (petroleum ether: acetone = 6:1 ~ 3:1) to obtain 480 mg of compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com