Medicine composition for treating metabolic bone diseases
A bone disease and drug technology, applied in the field of medicine, can solve problems such as inconvenient administration methods, and achieve the effects of convenient medication, convenience for patients, and good therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Effect of Minodronic Acid and Alfacalcidol / Calcitriol on Metabolic Bone Disease in Rats
[0028] (1), the method of administration
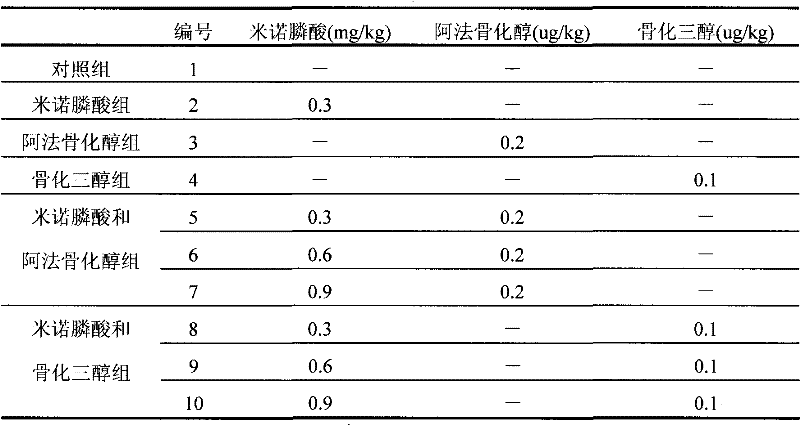
[0029] Take 110 healthy, clean, mature and roughly identical female rats, divide them into 11 groups, 10 rats in each group, keep one group as the blank group after one week of normal feeding, and the remaining 10 groups undergo ovariectomy, and take oral administration, respectively. It is: minodronic acid, alfacalcidol, calcitriol and their combination drugs are administered alone, and the administration time is 8 weeks, once a week. The dosage is shown in Table 1.
[0030] (2), determination method
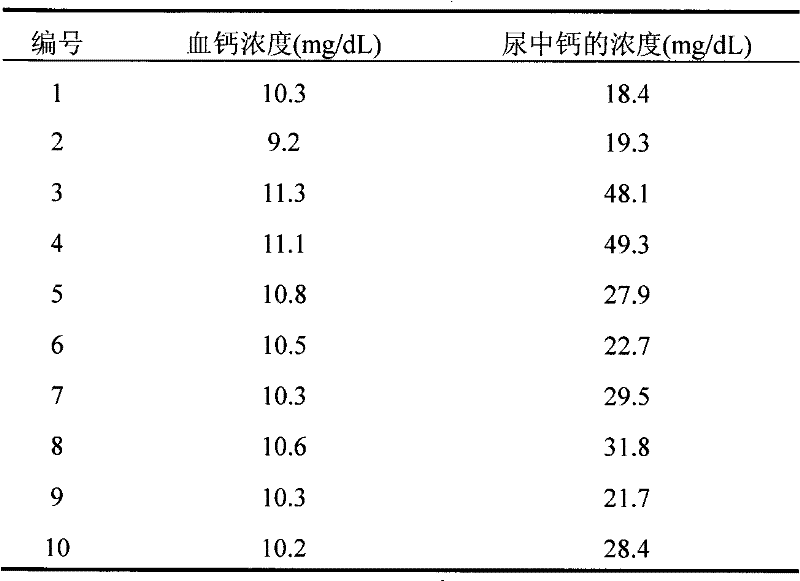
[0031] Urine samples from rats were collected for 24 hours, centrifuged, and stored at low temperature. Take blood, centrifuge, collect serum, and store at low temperature. Calcium determination kit (ARSENAZO III method) was used to measure blood calcium concentration and urinary calcium (24h) concentration.
[0032] Rat tibi...
Embodiment 2-3
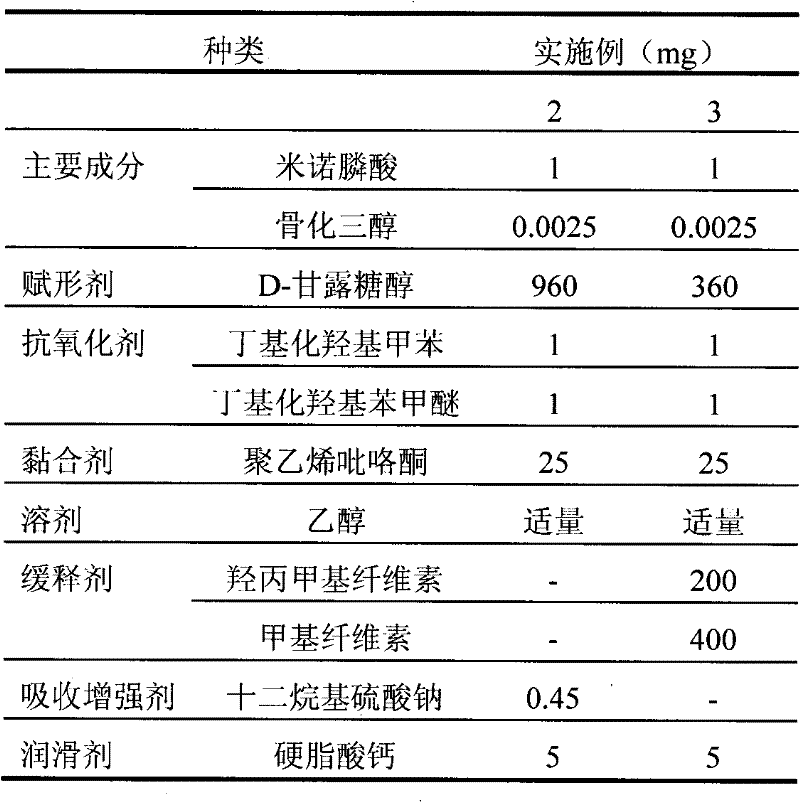
[0041] The preparation of embodiment 2-3 minodronic acid and calcitriol tablet
[0042] Prepare bare chips according to the composition and content shown in Table 3.
[0043] First, D-mannitol was added to a mixture of calcitriol and ethanol, followed by ethanol, antioxidants, and binders. Separately from the step of preparing calcitriol, the other ingredients minodronic acid, mannitol, absorption enhancer are mixed; ethanol and binder are then added thereto. The calcitriol and minodronic acid are then mixed, then a disintegrant and lubricant are added, and the tablet is compressed.
Embodiment 4-5
[0044] The preparation of embodiment 4-5 minodronic acid and alfacalcidol capsules
[0045] Capsules were prepared as shown in Table 4.
[0046] First, add xylitol to a mixture consisting of alfacalcidol and ethanol, then add ethanol, antioxidants and binders, use a conventional pulverizer to pulverize the whole mixture into 16-50 mesh size and dry at 40°C to obtain alfacalcidol granules. Separately from the step of preparing calcitriol, the other ingredients minodronic acid, xylitol, absorption enhancer are mixed; ethanol and binder are then added to the above mixture. The whole mixture was then pulverized into a 16-50 mesh size using a conventional pulverizer and dried at 40°C to obtain minodronic acid granules. The alfacalcidol and minodronic acid are then mixed, then a disintegrant and a lubricant are added, and the capsules are filled.
[0047] table 3
[0048]
[0049] Table 4
[0050]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap