Modulation of toll-like receptor 4 expression by antisense oligonucleotides
An antisense oligonucleotide and oligonucleotide technology, applied in the field of Toll-like receptor 4, can solve the problems of non-specificity, inactivity, toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Preparation of TLR4-specific antisense oligonucleotides
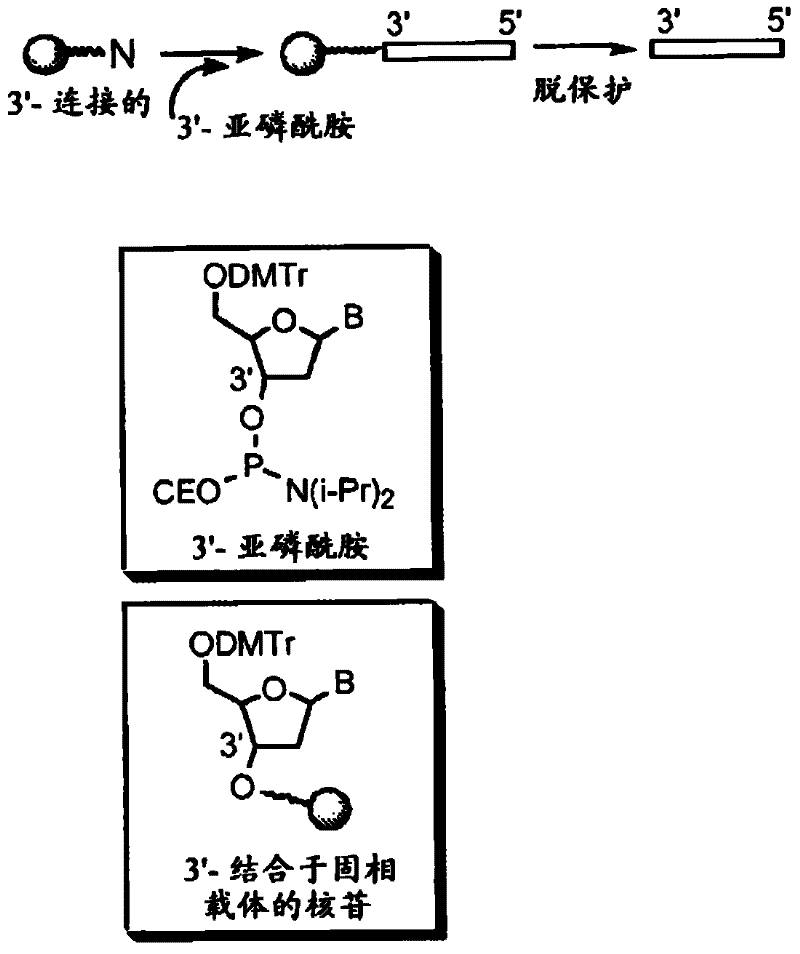
[0120] Using an automated DNA synthesizer (OligoPilot II, AKTA, (Amersham) and / or Expedite 8909 (Applied Biosystem)) followed figure 1 Chemical entities according to the invention were synthesized on a 1 μmol to 0.1 mM scale using the linear synthetic protocol outlined in .
[0121] 5'-DMT dA, dG, dC and T phosphoramidites were purchased from Proligo (Boulder, CO). 5'-DMT 7-deaza-dG and araG phosphoramidites were obtained from Chemgenes (Wilmington, MA). DiDMT-glycerol linker solid phase support was obtained from Chemgenes. 1-(2'-deoxy-β-D-ribofuranosyl)-2-oxo-7-deaza-8-methyl-purine amidite was obtained from Glen Research (Sterling, VA), 2 '-O-methyl ribonucleoside imide was obtained from Promega (Obispo, CA). All compounds according to the invention are phosphorothioate backbone modified.
[0122] pass 31 P and 1 H NMR spectroscopy to characterize all nucleoside phosphoramidites. Modified nucleo...
Embodiment 2
[0124] Cell Culture Conditions and Reagents
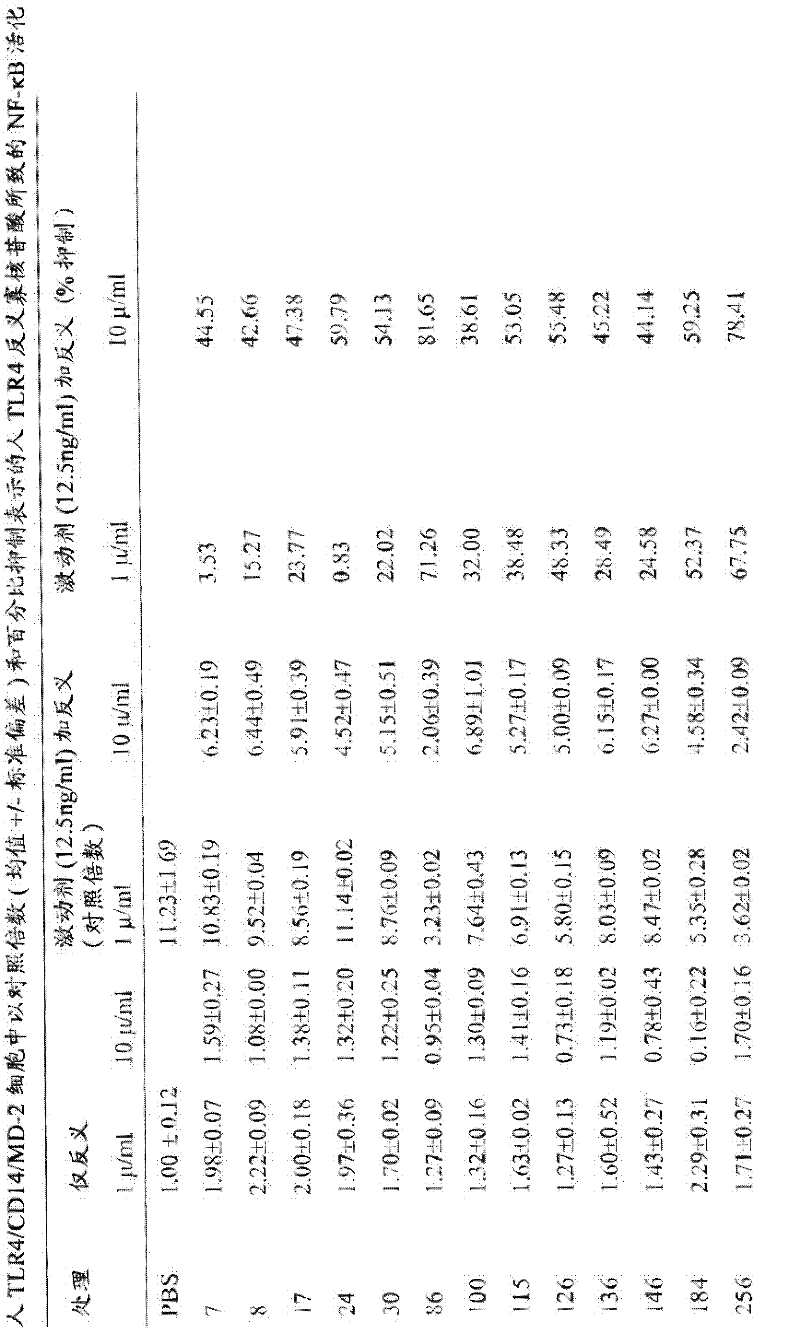
[0125] HEK293 Cell Culture Assay for TLR4 Antisense Activity
[0126] at 5% CO 2 HEK293 cells stably expressing human TLR4 / CD14 / MD-2 (Invivogen, San Diego, CA) were dispensed in 48-well plates at 250 μL / well in DMEM supplemented with 10% heat-inactivated FBS in an incubator. At 80% confluency, cells were treated with 400 ng / mL secreted human embryonic alkaline phosphatase (SEAP) reporter plasmid (pNifty2-Seap) (Invivogen) in the presence of 4 μL / mL Lipofectamine (Invitrogen, Carlsbad, CA) in culture medium. Transiently transfect cultures. The SEAP reporter plasmid can be induced by NF-κB. Plasmid DNA and Lipofectamine were diluted separately in serum-free medium and incubated at room temperature for 5 minutes. After incubation, the diluted DNA and Lipofectamine were mixed, and the mixture was incubated for an additional 20 minutes at room temperature. Aliquots of 25 µL of the DNA / Lipofectamine mixture containing 100 ng of pl...
Embodiment 3
[0129] In Vivo Activity of TLR4 Antisense Oligonucleotides
[0130] Subcutaneously inject 5 mg / kg of mouse TLR4 antisense oligonucleotide or PBS according to an example of the present invention to 5-6 week-old female C57BL / 6 mice (N=3 / group), once a day for 3 days . Following administration of TLR4 antisense oligonucleotides, mice were injected subcutaneously with 0.25 mg / kg TLR4 agonist. Two hours after TLR agonist administration, blood was collected and TLR-mRNA, TLR4 protein and IL-12 concentrations were determined by ELISA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com