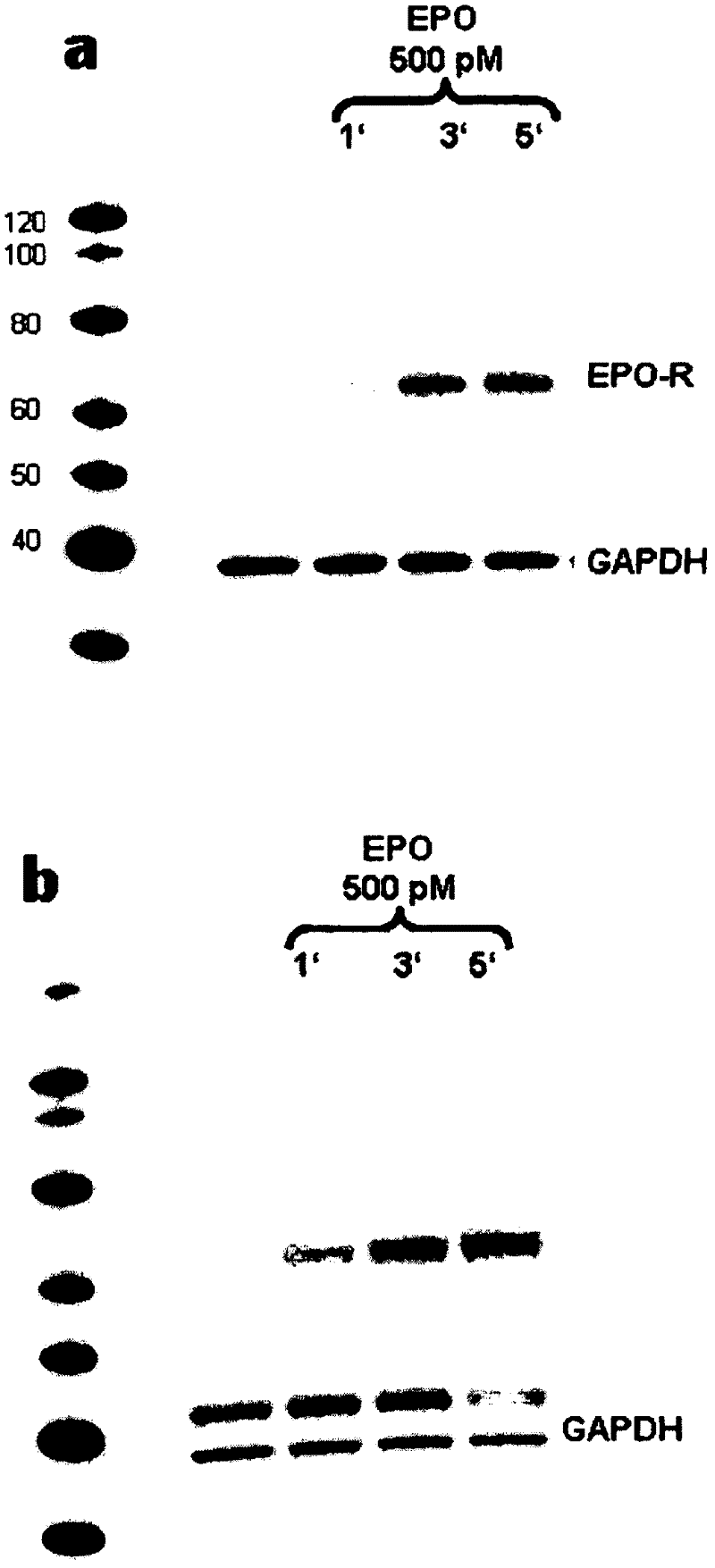
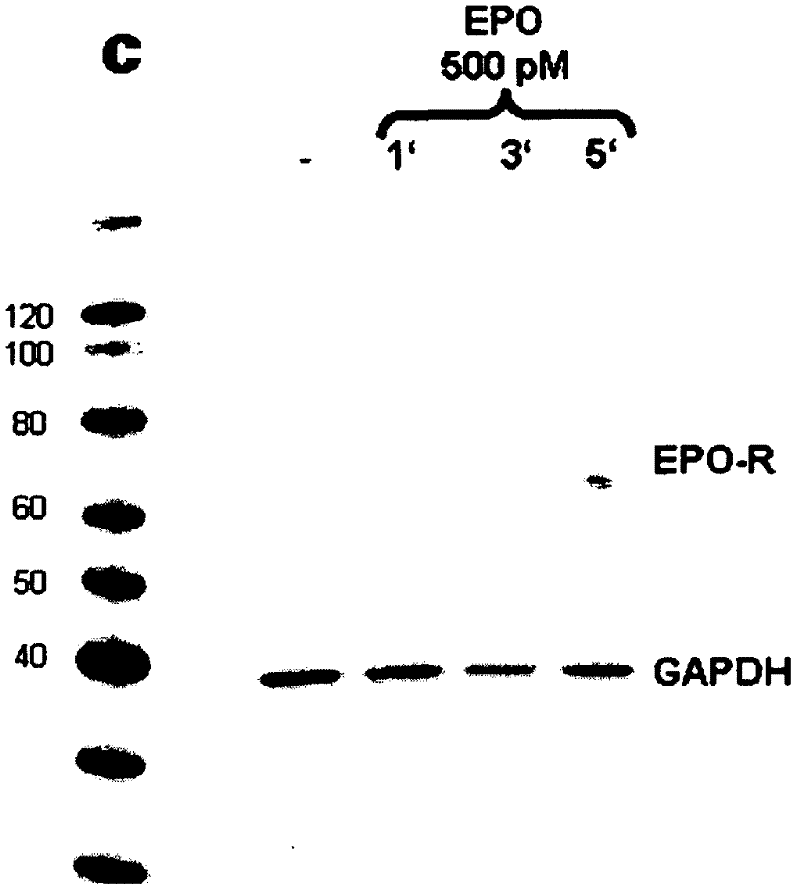
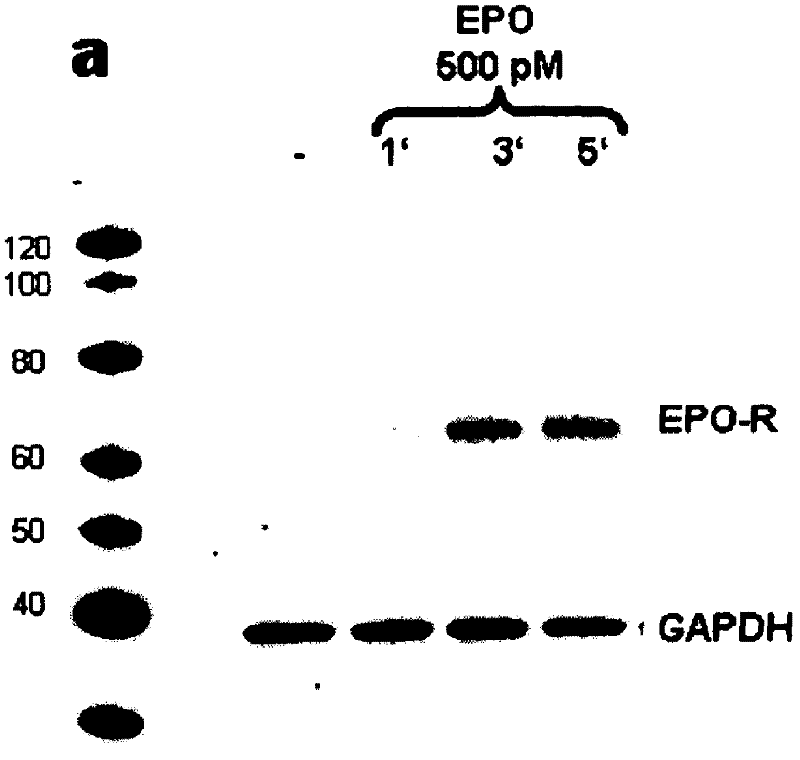
Antibody to human epo receptor
By developing antibodies that specifically bind to phosphotyrosyl residues, the problem that existing technology cannot distinguish between non-activated and activated EPO-R is solved, accurate analysis of the activation status of EPO-R is achieved, and diagnosis and research are improved. accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The term "antibody" encompasses various forms of antibody structures including, but not limited to, whole antibodies and antibody fragments.
[0028] An "antibody fragment" comprises a portion of a full-length antibody, preferably its variable domain, or at least its antigen-binding site. Examples of antibody fragments include diabodies, single chain antibody molecules, and multispecific antibodies formed from antibody fragments. scFv antibodies are described, eg, in Houston, J.S., Methods Enzymol. 203 (1991) 46-88. In addition, antibody fragments contain V H domain (i.e. capable of interacting with V L domain co-assembly) or V that binds EPO-R L domain (i.e. capable of interacting with V H Domains are assembled together as a single-chain polypeptide characteristic of a functional antigen-binding site), thereby providing an antibody with specific binding properties to human EPO-R.
[0029]The term "humanized antibody" refers to an antibody whose framework and / or "c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition ratio | aaaaa | aaaaa |
| composition ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com