RNA interference-mediated suppression of epithelial sodium channel (ENAC) gene expression using short interfering nucleic acid (SINA)
A technology for interfering nucleic acids and nucleotides, which is applied in the field of RNA interference-mediated inhibition of epithelial sodium channel (ENaC) gene expression using short interfering nucleic acids (siNA), and can solve problems such as reduced RNAi activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0885] Synthetic methods used for RNA comprising certain siNA molecules of the invention follow procedures as described in the following references: Usman et al., 1987, J.Am.Chem.Soc., 109, 7845; Scaringe et al., 1990, Nucleic Acids Res., 18, 5433; and Wincott et al., 1995, Nucleic Acids Res.23, 2677-2684 Wincott et al., 1997, Methods Mol. Bio., 74, 59, and utilize common nucleic acid protection and conjugation Groups such as dimethoxytrityl at the 5'-end, and phosphoramidites at the 3'-end. In a non-limiting example, small-scale synthesis was performed on a 394 Applied Biosystems, Inc. synthesizer using a 0.2 μmol scale protocol with a 7.5-minute coupling step for alkylsilyl-protected nucleotides and a 7.5-minute coupling step for A 2.5 minute coupling step was used for 2'-O-methylated nucleotides. Table 9 summarizes the amounts of reagents and contact times used in the synthesis cycle. Alternatively, synthesis at the 0.2 μmol scale can be performed on a 96-well plate synth...
Embodiment 1
[0992] Example 1: Design, synthesis and identification of siNAs active against ENaCα
[0993] Synthesis of ENaCα siNA
[0994] A series of 64 siNA strands were designed, synthesized and evaluated for efficacy against ENaC. The main criteria for ENaC siNA design were (i) conservation of ENaC across human, mouse and rat isoforms, and (ii) high efficacy score as determined by a proprietary algorithm. The effect of siNA on ENaC protein production and RNA levels was also examined. The siNA sequences designed, synthesized and evaluated for efficacy against ENaC are described in Table 1a (target sequences) and Table 1b (modified sequences).
[0995] Table 1a: ENaCα target sequences, target sites indicated.
[0996]
[0997]
Embodiment 2
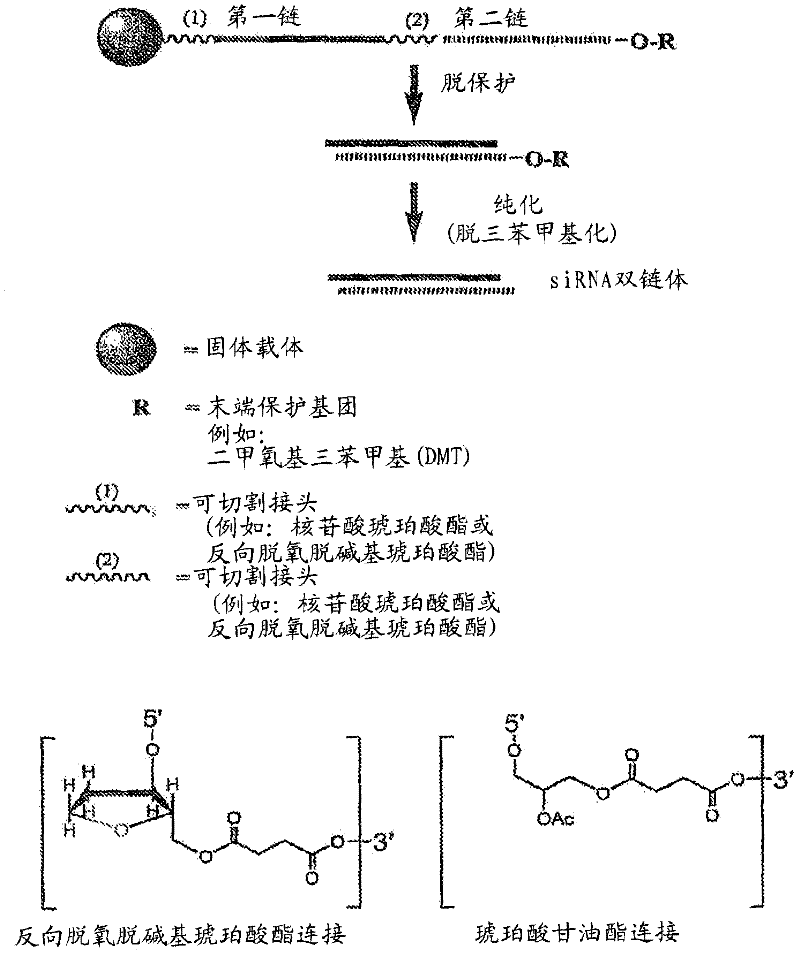
[0998] Example 2: Tandem Synthesis of siNA Constructs
[0999] Exemplary siNA molecules of the invention are synthesized in tandem using cleavable linkers, such as succinyl-based linkers. Tandem synthesis as described herein is followed by a single-step purification process that provides RNAi molecules in high yield. This method is highly amenable to siNA synthesis supporting high-throughput RNAi screening and can be easily adapted to multi-column or multi-well synthesis platforms.
[1000] After tandem synthesis of siNA oligonucleotides (oligo) and their complements in which the 5′-terminal dimethoxytrityl (5′-O-DMT) remains intact (trityl-dependent synthesis), the oligonuclear The nucleotides were deprotected as described above. After deprotection, the siNA sequence strands are allowed to hybridize spontaneously. This hybridization produces a duplex in which one strand has retained a 5'-O-DMT group and the complementary strand contains a terminal 5'-hydroxyl. The newly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap