Aminated hemicellulose molecule and method for production thereof
A kind of hemicellulose, amination technology, applied in the direction of amino sugar, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
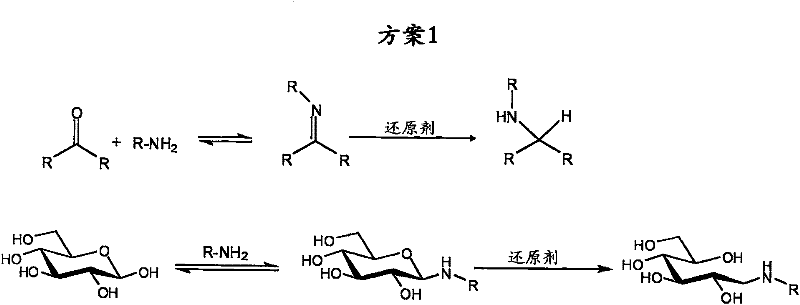
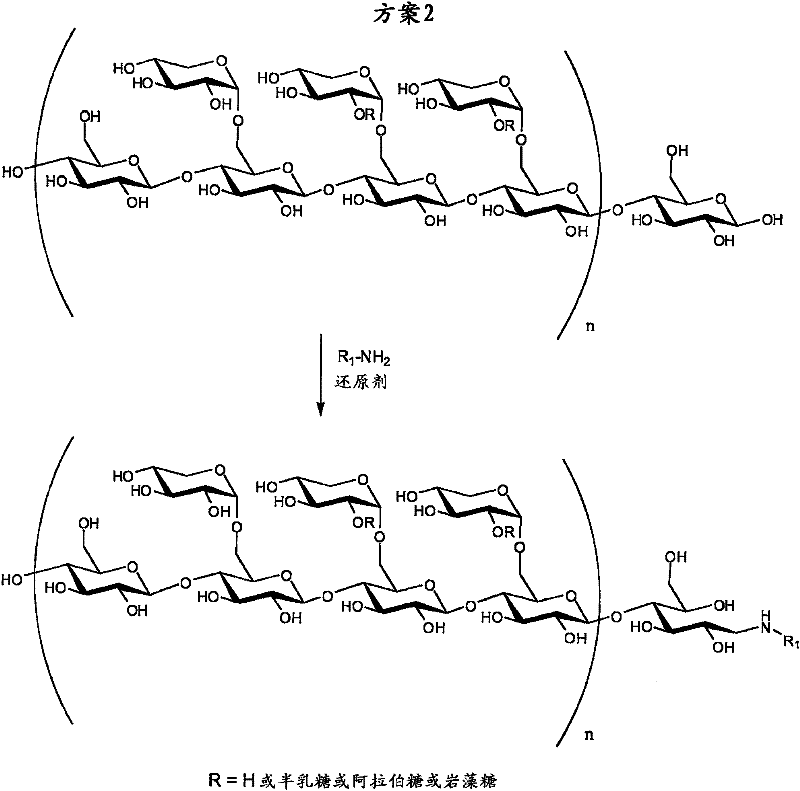
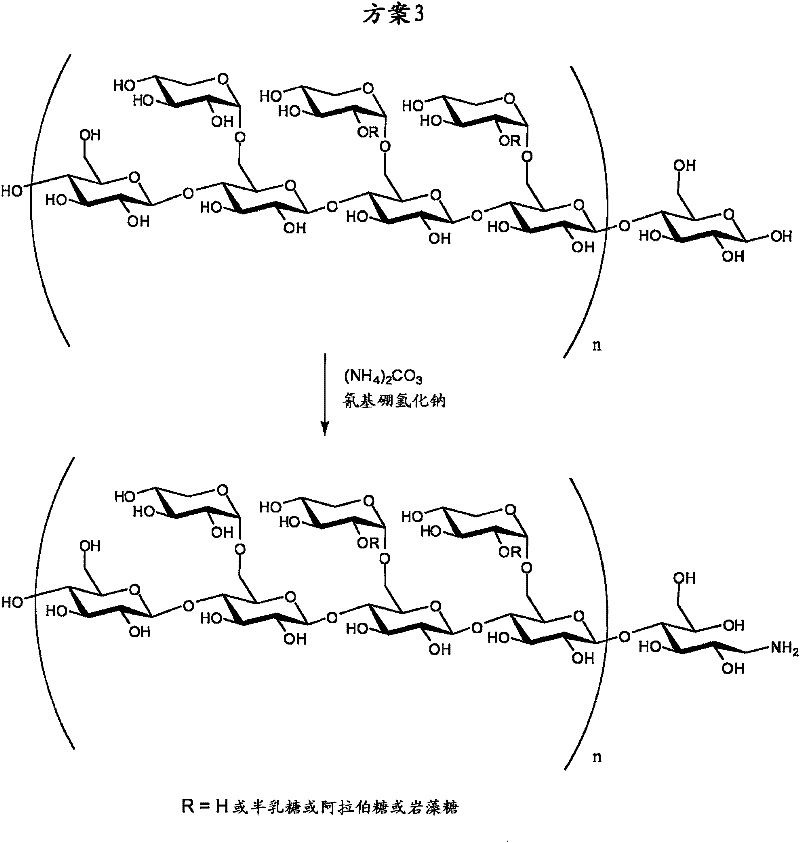
[0132] used XGO and reacted with sodium cyanoborohydride as reducing agent, scheme 2
Embodiment 1
[0134] R 1 = Benzyl (Bn)
[0135] Dissolve XGO (50 g, 39 mmol) in MeOH / H at 55 °C 2 O (4:1, 700 mL). Benzylamine (6.4 mL, 59 mmol) and NaCNBH were added 3 (3.7 g, 59 mmol). Acetic acid (7 mL) was then added to bring the pH to 5 and the reaction mixture was stirred overnight at 55°C. According to TLC (acetonitrile / H 2 (2:1) and MALDI-TOF confirmed that the reaction mixture was evaporated to remove methanol after the completion of the reaction. The product was then precipitated in ice ethanol (3 L) and the white solid was collected by centrifugation. The product was dried under vacuum to obtain 49 g of product (36 mmol, 92%). (R 1 =Bn).
Embodiment 2
[0137] R 1 = Allyl (All)
[0138] Dissolve XGO (3 g, 2.34 mmol) in 25 mL MeOH, 5 mL H 2 O and 0.30mL acetic acid mixture. Join NaCNBH 3 (0.220 g, 3.5 mmol) and allylamine (0.277 mL, 3.69 mmol), the reaction was stirred overnight at 55°C. According to TLC (acetonitrile / H 2 (2:1) and MALDI-TOF to determine the reaction mixture was precipitated in ice ethanol. The white material was collected by centrifugation, dissolved in water and concentrated. Freeze drying afforded 2.65 mg of product (0.20 mmol, 85%). (R 1 =All).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com