Compositions for continuous administration of dopa decarboxylase inhibitors
A kind of technology of composition and liquid composition, applied in the field of arginine salt and composition containing them, can solve the problems such as never
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Preparation and characterization of embodiment 1 carbadopa-arginine salt
[0069] Carbidopa (CD)-arginine salt was prepared as follows:
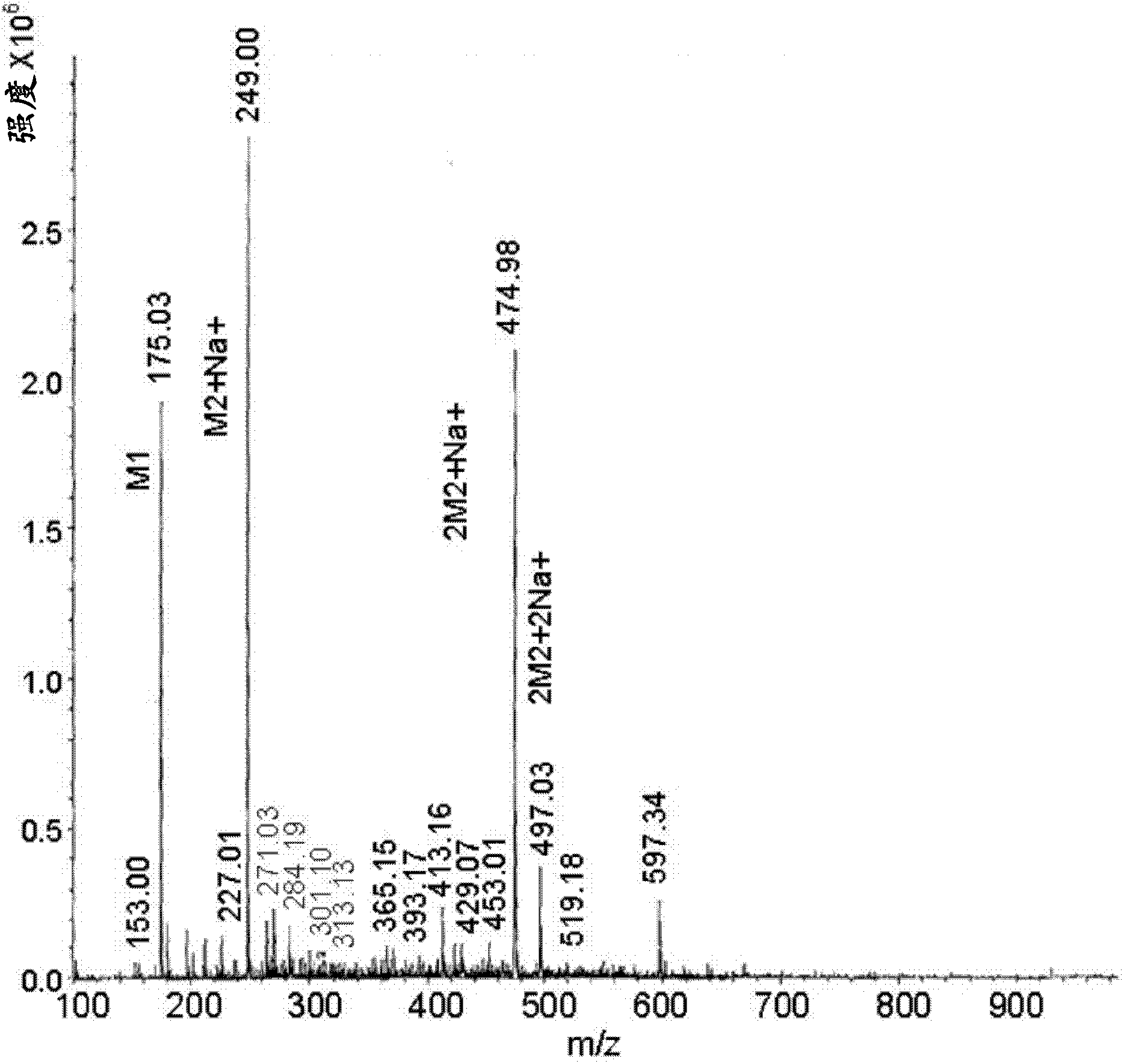
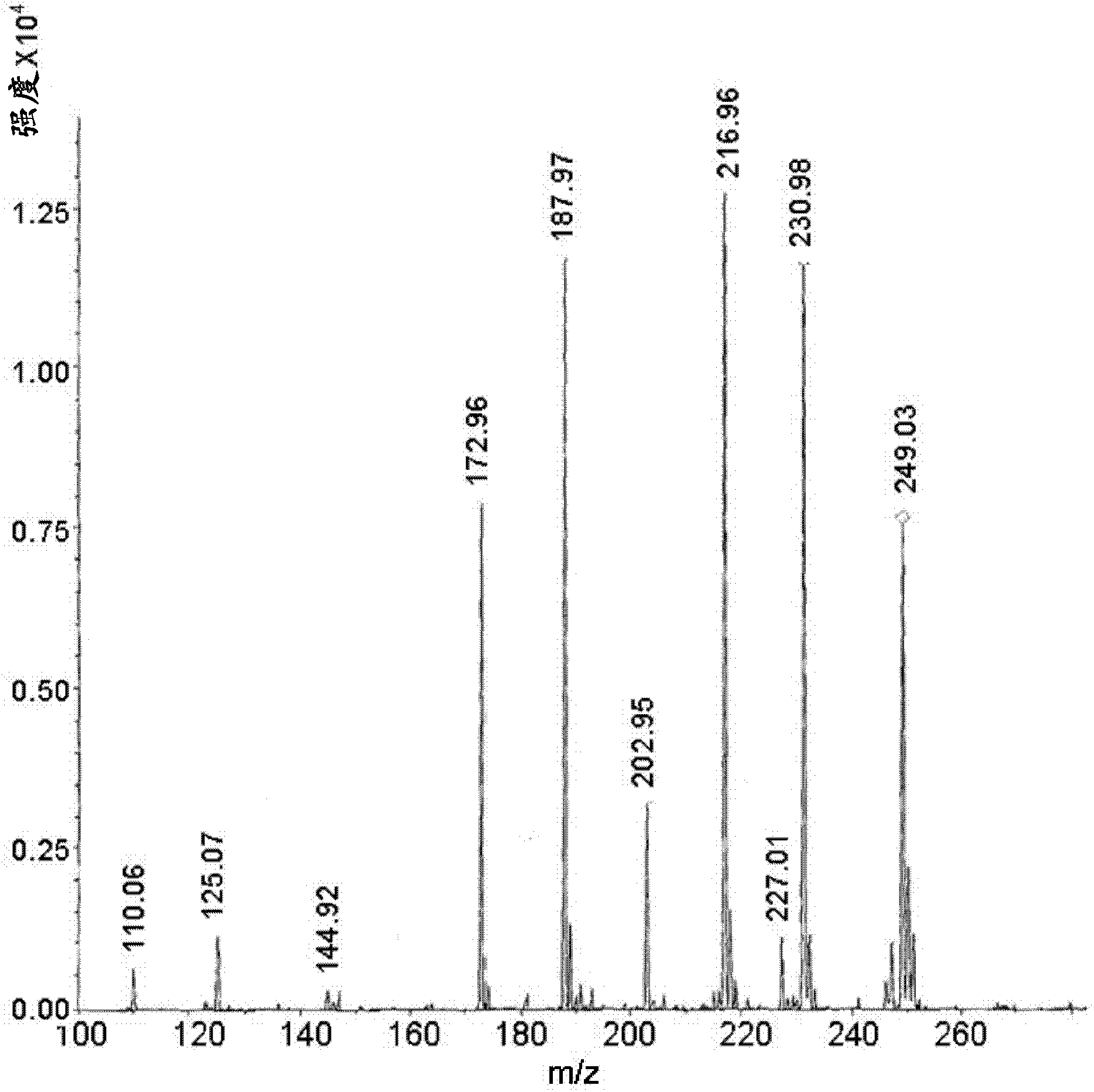
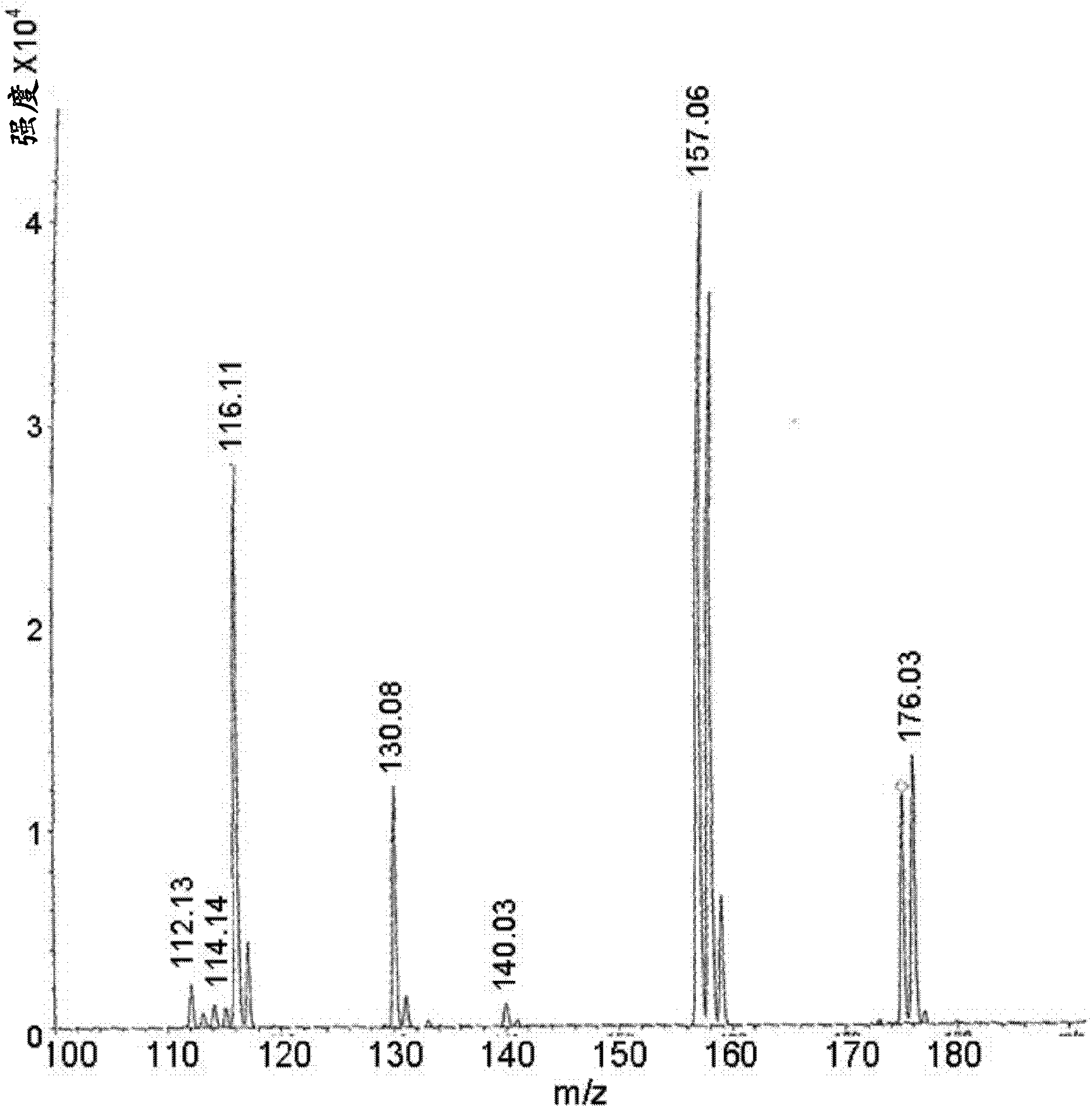
[0070] Weigh carbidopa [Teva Pharmaceuticals Ltd., Israel] and L-arginine [Merck] (in a 1:1 molar ratio) into a suitable container and add 0.2% aqueous sodium bisulfite [Sigma] A final concentration of 4.0% carbidopa was obtained. The mixture was heated to 65±10°C with constant stirring. When the solids were completely dissolved, the solution was filtered using a 0.45 μM nylon membrane. The filtered solution was immediately frozen on dry ice and then freeze-dried. Off-white crystals were obtained which were then subjected to MS analysis. MS analysis results clearly showed carbidopa and L-arginine ions and fragments (Fig. 1a). Peak 249 represents carbidopa+Na(226+23) with fragments: 227, 188 and 144 (Figure 1b); peak 176 represents arginine+2H(174+2) with fragments 157, 130 and 116 ( Figure 1c).
Embodiment 2
[0071] Example 2 Preparation of carbidopa solution / formulation for subcutaneous administration
[0072] A 4% carbidopa solution / formulation was prepared as follows:
[0073] Carbidopa [Assia Ltd., Israel] claims to reconstitute in a suitable container and then add water to give 73% of the total estimated batch weight. The mixture was stirred at room temperature for 20 minutes. L-Arginine [Sigma] was added to the mixture to give a 1:1 molar ratio to carbidopa. The mixture was heated to 65±10°C with constant stirring. When the solid was completely dissolved, N-methyl 2-pyrrolidone [Pharmasolve, ISP] was added to give a final concentration of 10% (w / w). Sodium bisulfite [Sigma] solution was prepared and added to give a final concentration of 1% (v / w). Stirring was continued for another 30 minutes at 65±3°C. Afterwards, a PVP [polyvinylpyrrolidone, Sigma] solution was prepared and added to obtain a final concentration of 1% (v / w). Stirring was continued for 30 minutes at 65±...
Embodiment 3
[0075] Example 3 - Preparation of carbidopa solutions / formulations for subcutaneous administration
[0076] A 6% carbidopa solution / formulation was prepared as follows:
[0077] Carbidopa [Teva Pharmaceuticals Ltd, Israel] and L-arginine [Merck] (molar ratio 1:1.1) were weighed in a suitable container and water was then added to give a total estimated batch weight of 84%. N-methyl 2-pyrrolidone [Pharmasolve, ISP] was added to give a final concentration of 5% (w / w). Sodium bisulfite [Sigma] solution was prepared and added to give a final concentration of 0.1% (v / w). The mixture was heated to 65±10°C with constant stirring. Heating was stopped when the solid had completely dissolved and the preparation was allowed to cool to room temperature. The solution was filtered using a sterile 0.22 μM PVDF membrane.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap