UV-curable optical resin adhesive composition
A technology of optical resins and adhesives, applied in the directions of optics, non-polymer organic compound adhesives, adhesives, etc., to achieve the effects of improved coating, improved chemical resistance, and reduced viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
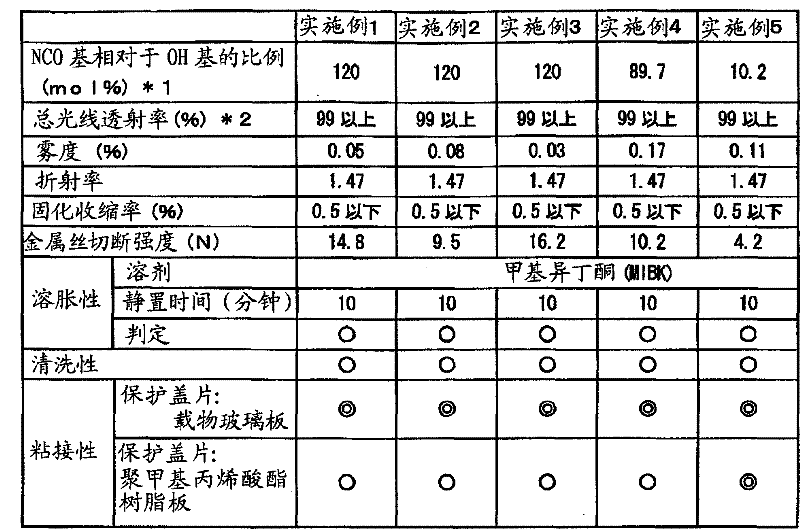
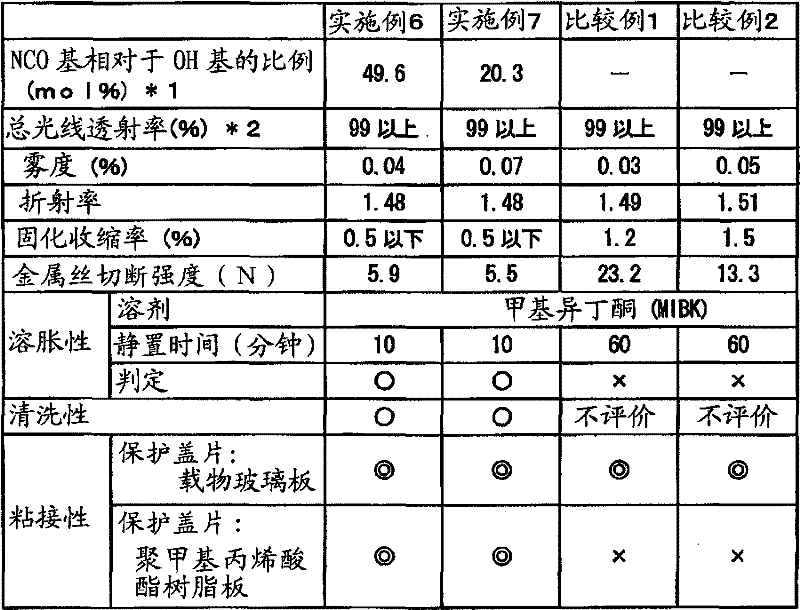
[0057] 40g (0.0784mol in terms of OH groups) as a vinyl polymer containing hydroxyl groups, a liquid acrylic polymer with a viscosity of 6000mPa·s, a weight average molecular weight of 2000, and a hydroxyl equivalent (OHV) of 110mgKOH / g, 14.6g (0.0941mol ) 2-isocyanatoethyl methacrylate was put into the reaction vessel respectively, and kept at 50° C. in a water bath under nitrogen flow. Then, 0.028 g (0.19 weight% with respect to 2-isocyanatoethyl methacrylate) of dibutyltin dilaurate (catalyst) was added, and it was kept for 8 hours, and it reacted. Then, using infrared absorption spectroscopy (FT-IR: manufactured by Thermo Electron Co., Ltd., FT-IR200 type), it was confirmed that the 2260 cm in the reaction product -1 (the characteristic absorption band originating from the isocyanate group) disappears. From this, it was found that an acrylic polymer (weight average molecular weight: 2100) having a methacryloyl group in the side chain was generated.
[0058] Next, 8 g of ...
Embodiment 2
[0060] 40g (0.0143mol in terms of OH groups) as a vinyl polymer containing hydroxyl groups, a liquid acrylic polymer with a viscosity of 14000mPa·s, a weight average molecular weight of 11000, and a hydroxyl equivalent (OHV) of 20mgKOH / g, 2.65g (0.0171mol ) 2-isocyanatoethyl methacrylate was put into the reaction vessel respectively, and kept at 50° C. in a water bath under nitrogen flow. Then, 0.006 g (0.23 weight% with respect to 2-isocyanatoethyl methacrylate) of dibutyltin dilaurate (catalyst) was added, and it was kept for 8 hours, and it reacted. Then, using infrared absorption spectroscopy (FT-IR: manufactured by Thermo Electron Co., Ltd., FT-IR200 type), it was confirmed that the 2260 cm in the reaction product -1 (the characteristic absorption band originating from the isocyanate group) disappears. From this, it was found that an acrylic polymer (weight average molecular weight: 12,000) having a methacryloyl group in the side chain was produced.
[0061] Next, excep...
Embodiment 3
[0063] Prepare 9.5 g of the acrylic polymer having a methacryloyl group in the side chain prepared in Example 2 above, 0.5 g of tetrahydrofurfuryl acrylate as a diluent, and 0.21 g of 1-hydroxy- Cyclohexyl-phenyl-ketone, 0.9 g bis(2,4,6-trimethylbenzoyl)phenylphosphine oxide. Except for this, it carried out similarly to Example 1, and prepared the target ultraviolet curable optical resin adhesive composition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap