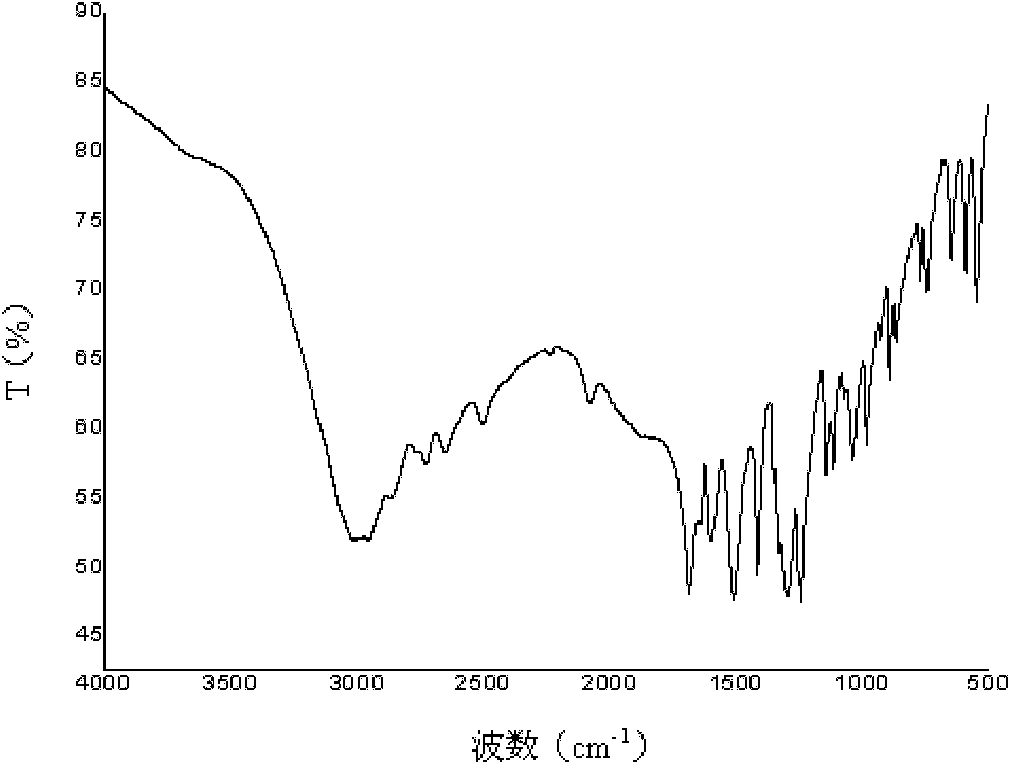
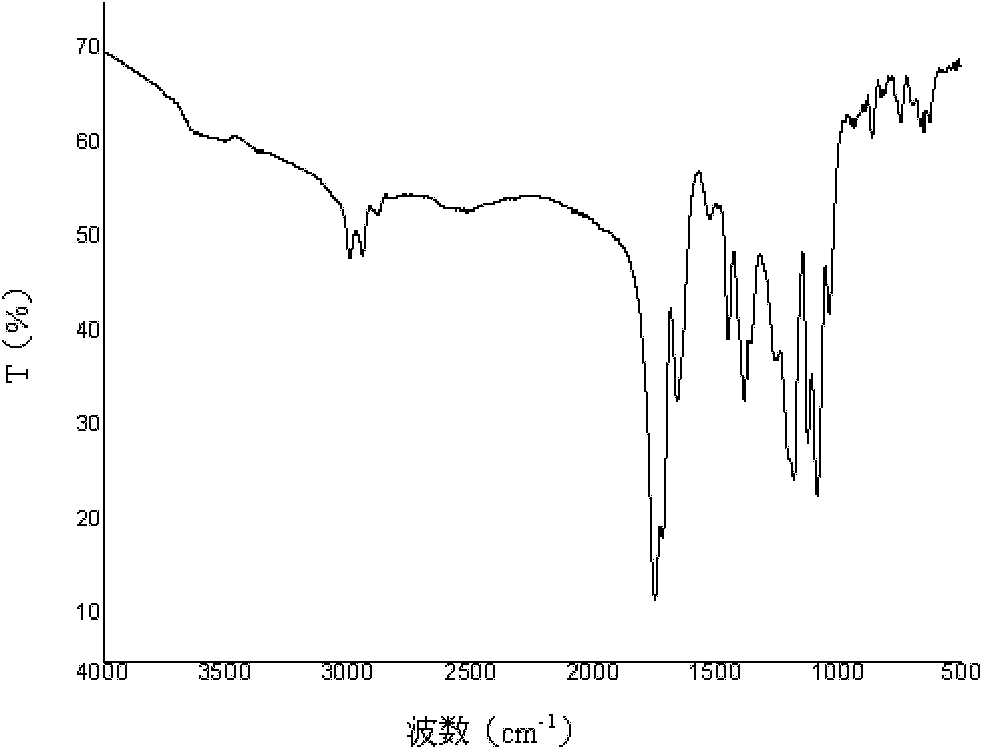
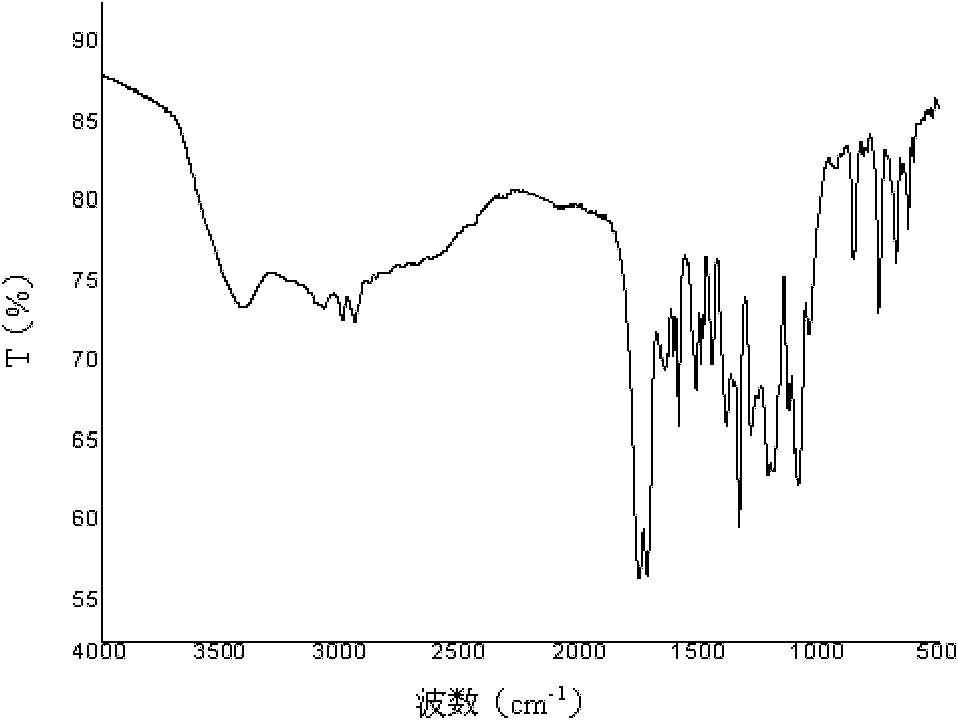
Poly(aspartic acid-co-lactic acid)-phosphatidyl ethanolamine graft polymer and preparation method and application thereof
A phosphatidylethanolamine and graft polymer technology, which is applied in the field of poly-phosphatidylethanolamine graft polymer and its preparation, can solve problems such as poor hydrophilicity, achieve strong lethality, improve bioavailability and biological activity, prolong the effect of the medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] (1) L-aspartic acid (3.33 grams, 0.025mol) (Alfar Aesar company, 98% by weight, analytically pure), L-lactide (7.2 grams, 0.05mol) (Alfar Aesar company, 97% by weight , analytically pure) was added in a 50-mL single-necked round-bottomed flask, vacuumed for 1 hour to remove oxygen, fed with nitrogen, and stirred and reacted in an oil bath at 180°C under nitrogen protection, the solution turned into a yellow transparent liquid. After 2.5 hours of reaction, the temperature was lowered to 160° C. for 21 hours of reaction, and the reaction liquid was a viscous light brown liquid. It was taken out from the oil bath and cooled to produce a tan solid, which was dissolved in 15 ml of N,N-dimethylformamide (Beijing Chemical Plant, analytically pure), and filtered to remove unreacted lactide. The filtrate was precipitated in 250 ml of deionized water and washed three times with 100 ml of deionized water. Dried in a vacuum oven at 25°C for 36 hours to obtain 8.4 grams of brown so...
Embodiment 2
[0097] (1) L-aspartic acid (3.33 grams, 0.025mol) (Alfar Aesar company, 98% by weight, analytically pure), L-lactide (7.2 grams, 0.05mol) (Alfar Aesar company, 97% by weight, Analytical grade) was added in a 50 ml single-necked round-bottomed flask, vacuumized for 1 hour to remove oxygen, fed with nitrogen, and stirred and reacted in an oil bath at 180°C under nitrogen protection, the solution turned into a yellow transparent liquid. After reacting for 3 hours, the temperature was lowered to 150° C. for 23 hours, and the reaction liquid was viscous light brown liquid. It was taken out from the oil bath and cooled to produce a tan solid, which was dissolved in 20 ml of N,N-dimethylformamide (Beijing Chemical Plant, analytically pure), and filtered to remove unreacted lactide. The filtrate was precipitated in 250 ml of deionized water and washed three times with 100 ml of deionized water. Dried in a vacuum drying oven at 20°C for 48 hours to obtain 8.9 grams of brown solid prod...
Embodiment 3
[0103] (1) L-aspartic acid (6.66 grams, 0.05mol) (Alfar Aesar company, 98% by weight, analytically pure), L-lactide (7.2 grams, 0.05mol) (Alfar Aesar company, 97% by weight , analytically pure) was added in a 100-mL single-necked round-bottomed flask, vacuumed for 1 hour to remove oxygen, fed with nitrogen, and stirred and reacted in an oil bath at 190°C under nitrogen protection, the solution turned into a yellow transparent liquid. After reacting for 5 hours, the temperature was lowered to 160° C. and reacted for 16 hours, and the reaction liquid was viscous light brown liquid. It was taken out from the oil bath and cooled to produce a tan solid, which was dissolved in 10 ml of N,N-dimethylformamide (Beijing Chemical Plant, analytically pure), and filtered to remove unreacted lactide. The filtrate was precipitated in 300 ml of deionized water and washed three times with 50 ml of deionized water. Dried in a vacuum oven at 30°C for 24 hours to obtain 11.1 grams of brown solid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com