Preparation of recombinant slow virus of shRNA (Short Hairpin Ribonucleic Acid) of target GPMV NP and M genes
A recombinant lentivirus and targeting technology, applied in genetic engineering, plant gene improvement, recombinant DNA technology, etc., can solve the problem of low transgenic efficiency and achieve wide application and far-reaching research effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Design of RNAi target and construction of shRNA lentiviral vector
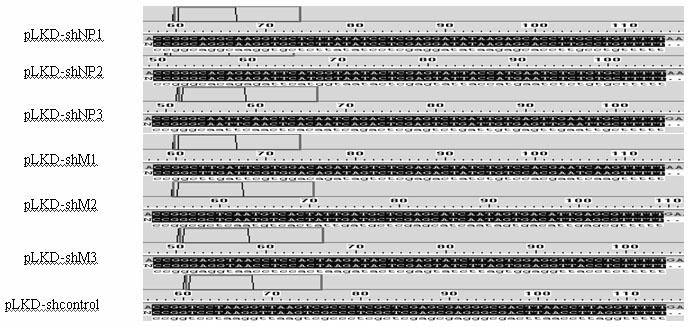
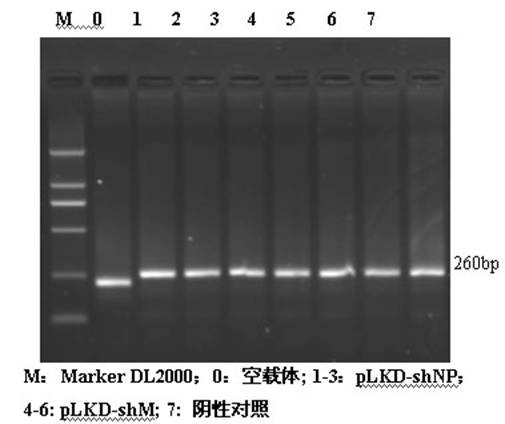
[0027] Using Ambion's siRNA online design software and design principles, screen the NP and M genes of the GPMV NA-1 strain for RNAi targets, and at the same time perform BLAST analysis on the obtained target sequences in GenBank; target siRNA oligonucleotides The sequence was sent to Shanghai Sangong for synthesis, and the synthesized oligonucleotide sequence was annealed to form a double strand, which was ligated with the linearized pLKD-EGFP vector after double digestion with endonuclease AgeⅠ and EcoRI, transformed into competent Escherichia coli DH5α, and picked for recombinant Positive clones were identified by PCR and sequencing (sequence analysis by Shanghai Sangon Company), and pLKD-shNP and pLKD-shM lentiviral vectors were produced.
[0028] The primer sequence of positive clones identified by PCR: F: 5'-AATGGACTATCATATGCTTACCGTAACTTG-3',
[0029] R: 5'-TGTCTGTTGCTATTATGTCTACTATTC...
Embodiment 2
[0030] Example 2 lentiviral packaging the
[0031] 24 hours before transfection, the 293T cells in the logarithmic growth phase were digested with 0.25% trypsin, and the cells were counted, and the cell density was adjusted to 5×10 6 cells / ml, reseeded in T75 cell culture flasks, and transfected when the cell density reached 70%-80%. Add the DNA solution of the plasmid in the lentiviral packaging system pLKD-shNP or pLKD-shM: 9μg; pCMV-dR8.2: 6.75μg and pCMV-VSV-G: 2.25μg to 500μl double antibody-free, serum-free DMEM culture medium and 54μl, mix well, and incubate at room temperature for 20min. Transfer the mixture of DNA and FuGENG HD transfection reagent to the culture medium of 293T cells and mix them evenly, culture them in a cell incubator at 37°C and 5% CO2, and discard the medium containing the transfection mixture after culturing for 6 hours. 12 ml of cell culture medium containing 10% FBS serum was added to the culture dish, and culture was continued for 48 hours...
Embodiment 3
[0032] Example 3 Determination of lentivirus titer by LaSRT method
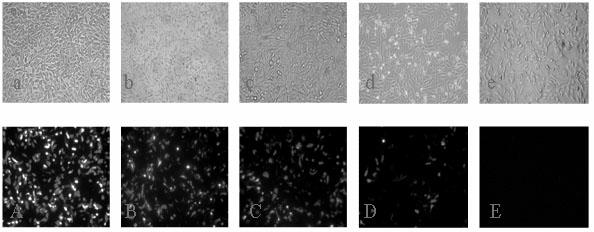
[0033] 293T cells with 2×10 4 Each well was inoculated in a 96-well culture plate, and after 12 hours, 8 wells were used as a group, and replaced with DMEM culture solution containing 8 μg / ml Polybrene and 10% FBS, 90 μl / well. Add 10 μl of the lentivirus stock solution to be tested into the first well, and then sequentially dilute each well 10:1 to set up and measure 3 groups of duplicate wells. After 48 hours of infection, observe the changes in the number of GFP-positive cells in each well under a fluorescent inverted microscope in the direction of concentration from high to low, determine the metering well (well n), and count the number of positive cells m, virus titer (TU / ml)=m×10 n+1 .
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap