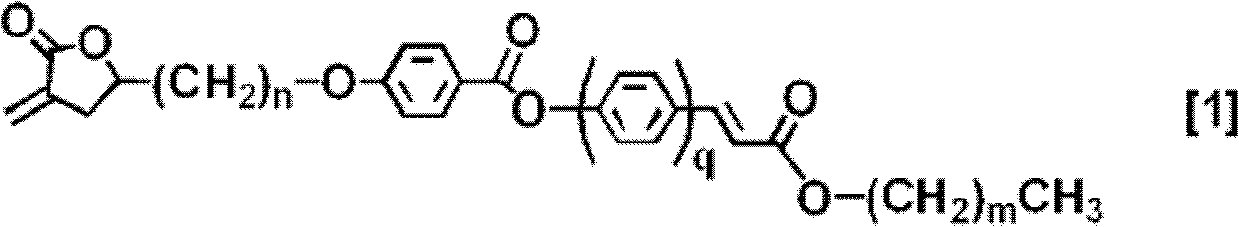
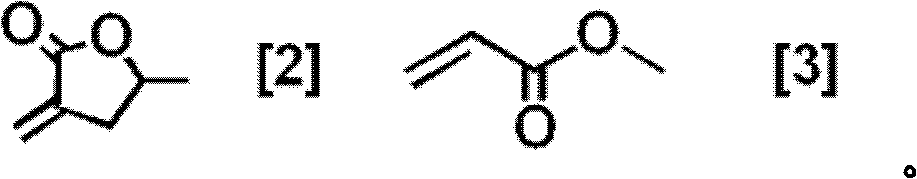
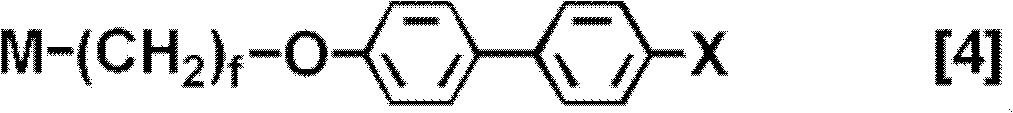
Polymerizable liquid crystal compound, polymerizable liquid crystal composition, and oriented film
一种聚合性液晶、液晶化合物的技术,应用在液晶材料、有机化学、非线性光学等方向,能够解决光学各向异性波长依赖性低等问题,达到低波长依赖性、高耐化学品性、高光学各向异性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0174] The present invention is described more specifically by way of Synthesis Examples, Examples and Comparative Examples, but the present invention should not be construed as being limited to the following Examples. It should be noted that the measurement methods and measurement conditions of the respective physical properties in the examples are as described below.
[0175] [1] NMR
[0176] The compound was dissolved in deuterated chloroform (CDCl 3 ) or deuterated dimethyl sulfoxide (DMSO-d6), use a nuclear magnetic resonance device (manufactured by Diol) to measure at 300MHz 1 H-NMR.
[0177] [2] Observation of liquid crystal phase
[0178] The liquid crystal phase was identified in such a manner that the sample was heated on a hot stage (MATS-2002S, manufactured by Tokai hit Co.), followed by observation by a polarizing microscope (manufactured by Nikon Corporation). The phase transition temperature was measured at a scan rate (Scan Rate) of 10° C. / min using a diffe...
Synthetic example 1
[0185] Synthesis of Polymeric Liquid Crystal Compound (E1)
[0186] [chemical formula 39]
[0187]
[0188] To a 500-ml round-bottomed flask equipped with a condenser was added 9.8 g (50.0 mmol) of 4-cyano-4'-hydroxybiphenyl, 7.0 g of 3-bromo-1-propanol (50.0 mmol), 13.8 g ( 100 mmol) of potassium carbonate and 150 ml of acetone to provide a mixture, followed by a reaction at 64° C. for 48 hours with stirring. After the reaction was completed, the solvent was distilled off under reduced pressure to obtain a yellow wet solid. Then, the solid was mixed together with 140 ml of water, and 100 ml of diethyl ether was added thereto for extraction. Repeat the extraction 3 times. The separated organic phase was dried by adding anhydrous magnesium sulfate and filtered, and then the solvent was distilled off under reduced pressure to obtain a yellow solid. The solid was purified by recrystallization from a mixed solvent of hexane / ethyl acetate=2 / 1 to obtain 8.7 g of a white solid...
Synthetic example 2
[0195] Synthesis of Polymeric Liquid Crystal Compound (E2)
[0196] [chemical formula 41]
[0197]
[0198] Add 5.0 g (25.6 mmol) of 4-cyano-4'-hydroxybiphenyl, 4.6 g (25.6 mmol) of 6-bromo-1-hexanol, 7.0 g ( 50mmol) of potassium carbonate and 50ml of acetone to provide a mixture, followed by a reaction at 64°C for 24 hours with stirring. After the reaction was completed, the solvent was distilled off under reduced pressure to obtain a yellow wet solid. Then, the solid was mixed together with 70 ml of water, and 50 ml of diethyl ether was added thereto for extraction. Repeat the extraction 3 times.
[0199] The separated organic phase was dried by adding anhydrous magnesium sulfate and filtered, and then the solvent was distilled off under reduced pressure to obtain a yellow solid. This solid was dissolved in 3 ml of ethyl acetate and subjected to silica gel column chromatography (column: silica gel 60, 0.063-0.200 mm, manufactured by Merck KGaA, eluent: hexane / ethyl ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hazing | aaaaa | aaaaa |
| hazing | aaaaa | aaaaa |
| hazing | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com