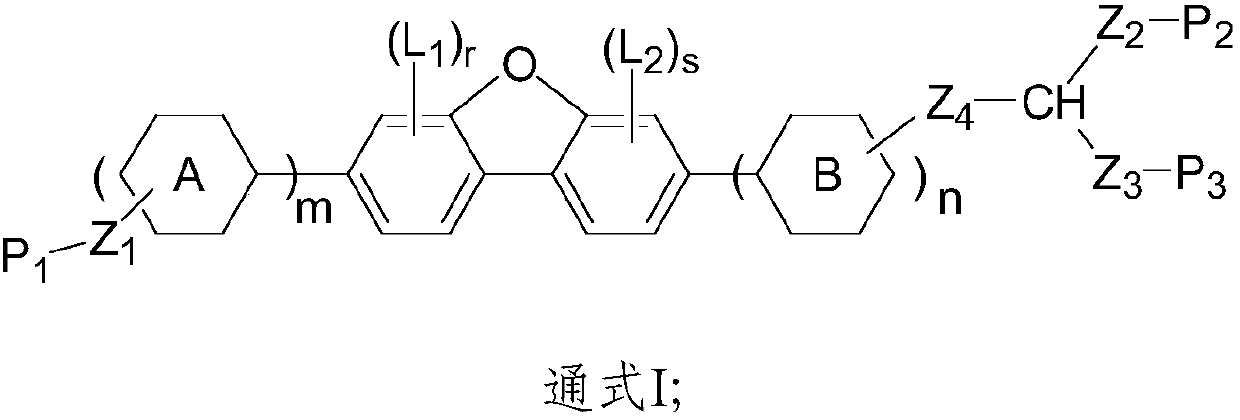
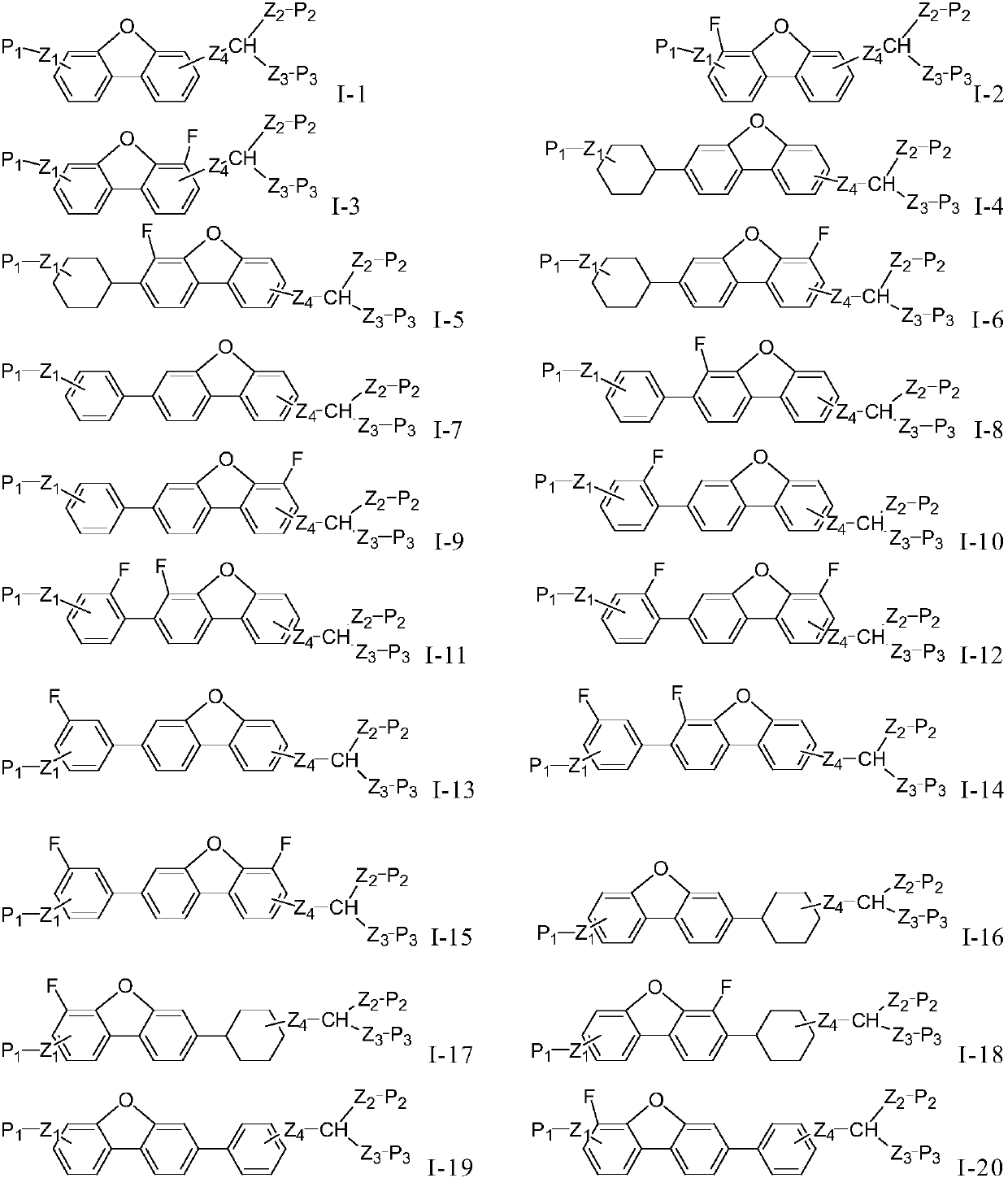
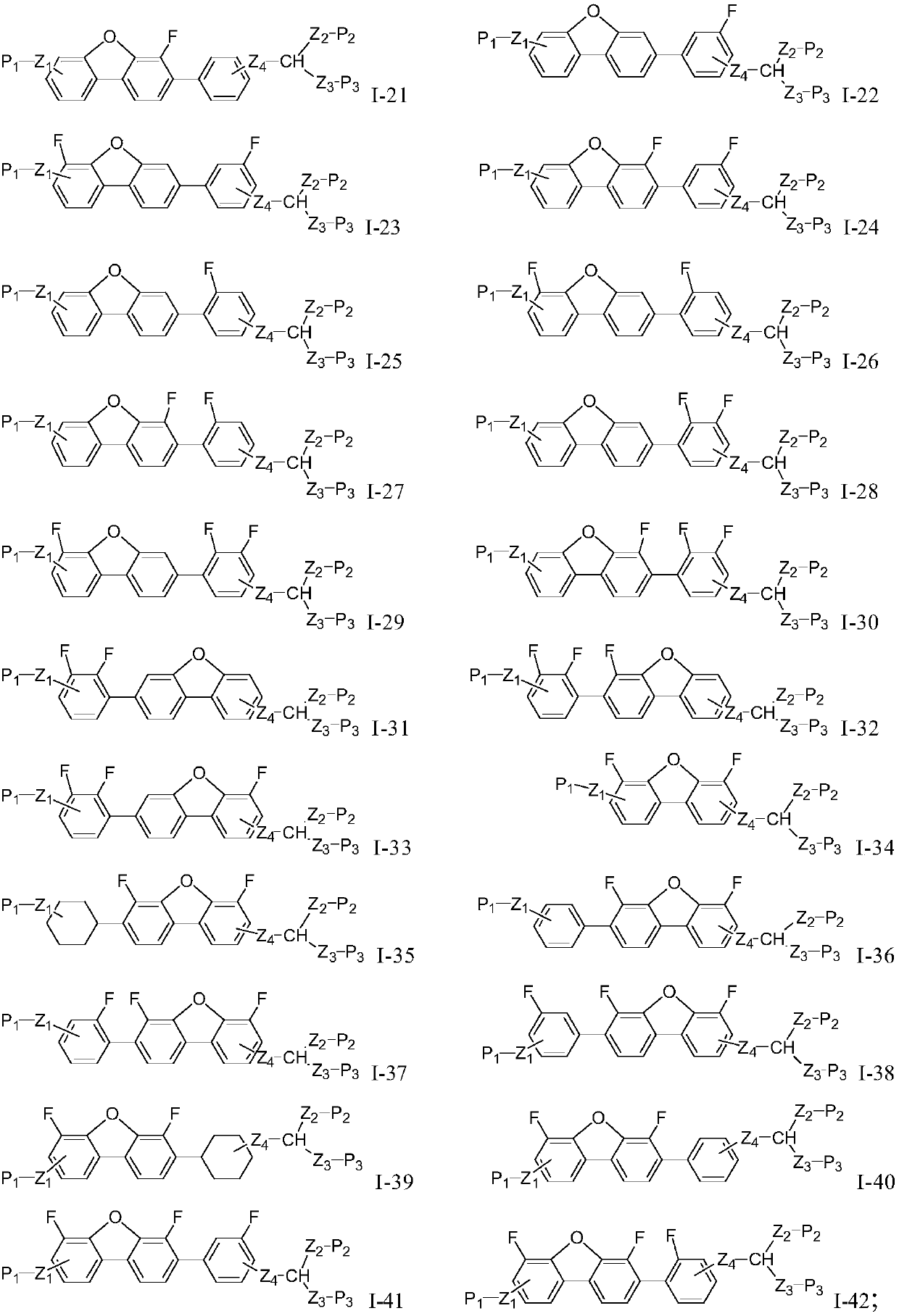
Dibenzofuran polymerizable compound and application thereof
A technology of dibenzofuran and compound, applied in the field of dibenzofuran polymerizable compounds, can solve the problems of poor homogeneity of polymerizable components, short UV sensitivity wavelength and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The structural formula of the liquid crystal compound is:
[0058]
[0059] The synthetic route for preparing compound BYLC-01 is as follows:
[0060]
[0061] Specific steps are as follows:
[0062] (1) Synthesis of compound BYLC-01-1:
[0063] 100ml N,N-dimethylformamide, 30g (0.087mol) was added to a 500ml reaction flask, 4.2g of sodium hydroxide was slowly added, the temperature was controlled to 45-55°C, and the reaction was carried out for 1h. Continue to control the temperature at 45-55° C., add 14.8 g of methyl iodide dropwise, and react at the temperature for 6 hours. The reaction solution was poured into water, filtered, washed until neutral, and dried to obtain 28.4 g of a white solid (compound BYLC-01-1, 0.079 mol), GC: 99.8%, yield: 90.8%.
[0064] (2) Synthesis of compound BYLC-01-2:
[0065] Under nitrogen protection, add 28.4g (0.079mol) compound BYLC-01-1, 25.2g diethyl malonate, 74.1g cesium carbonate, 1g cuprous iodide, 500ml tetrahydrofur...
Embodiment 2
[0075] The structural formula of the liquid crystal compound is:
[0076]
[0077] The synthetic route for preparing compound BYLC-02 is as follows:
[0078]
[0079] Specific steps are as follows:
[0080] Synthesis of compound BYLC-02:
[0081] Under the protection of nitrogen, add 30.0g of compound BYLC-01-3, 37.6g of triethylamine and 250mL of dichloromethane into the reaction flask, cool down to -10°C, control the temperature from -10°C to 0°C, and add 32.0g of acryloyl chloride dropwise, Rise to room temperature and react for 6 hours. Pour the reaction solution into water, neutralize it with aqueous uranium bicarbonate solution, perform conventional post-treatment, purify by chromatography, elute with n-hexane, and recrystallize from ethanol to obtain 39.4 g of a white solid (compound BYLC-02), LC : 99.6%, yield: 84.3%.
[0082] The obtained white solid BYLC-02 was analyzed by GC-MS, and the m / z of the product was 456.1 (M+).
[0083] 1 H-NMR (300MHz, CDCl 3 ...
Embodiment 3
[0085] The structural formula of the liquid crystal compound is:
[0086]
[0087] The synthetic route for preparing compound BYLC-03 is as follows:
[0088]
[0089] Specific steps are as follows:
[0090] (1) Synthesis of compound BYLC-03-1:
[0091] Under the protection of nitrogen, add 40g of 3-benzyloxy-7-bromo-4,6-difluorodibenzofuran and 300ml of tetrahydrofuran into the reaction flask, cool down to -70℃~-80℃, add dropwise 0.13mol of n-butyl Lithium, temperature control -70℃~-80℃, react for 1h, add 18.0g trimethyl borate dropwise, naturally return to -30℃, acidify with dilute hydrochloric acid to adjust the pH value to less than 2, carry out conventional post-treatment, and recrystallize to obtain light yellow Solid (compound BYLC-03-1) 31.2g, LC: 98.5%, yield 86.7%;
[0092] (2) Synthesis of compound BYLC-03-2:
[0093] Add 31.2g of compound BYLC-03-1, 17.5g of p-chlorobromobenzene, 18.0g of anhydrous potassium carbonate, 200ml of toluene, 150ml of ethanol, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com