Negative electrode, electrolytic cell for electrolysis of alkali metal chloride, and method for producing negative electrode
A manufacturing method and cathode technology, which are applied in the field of ion-exchange membrane salt electrolysis, can solve the problems of platinum shedding, lack of durability, and rise in electrolysis voltage, and achieve the effects of excellent resistance, excellent durability, and low hydrogen overvoltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
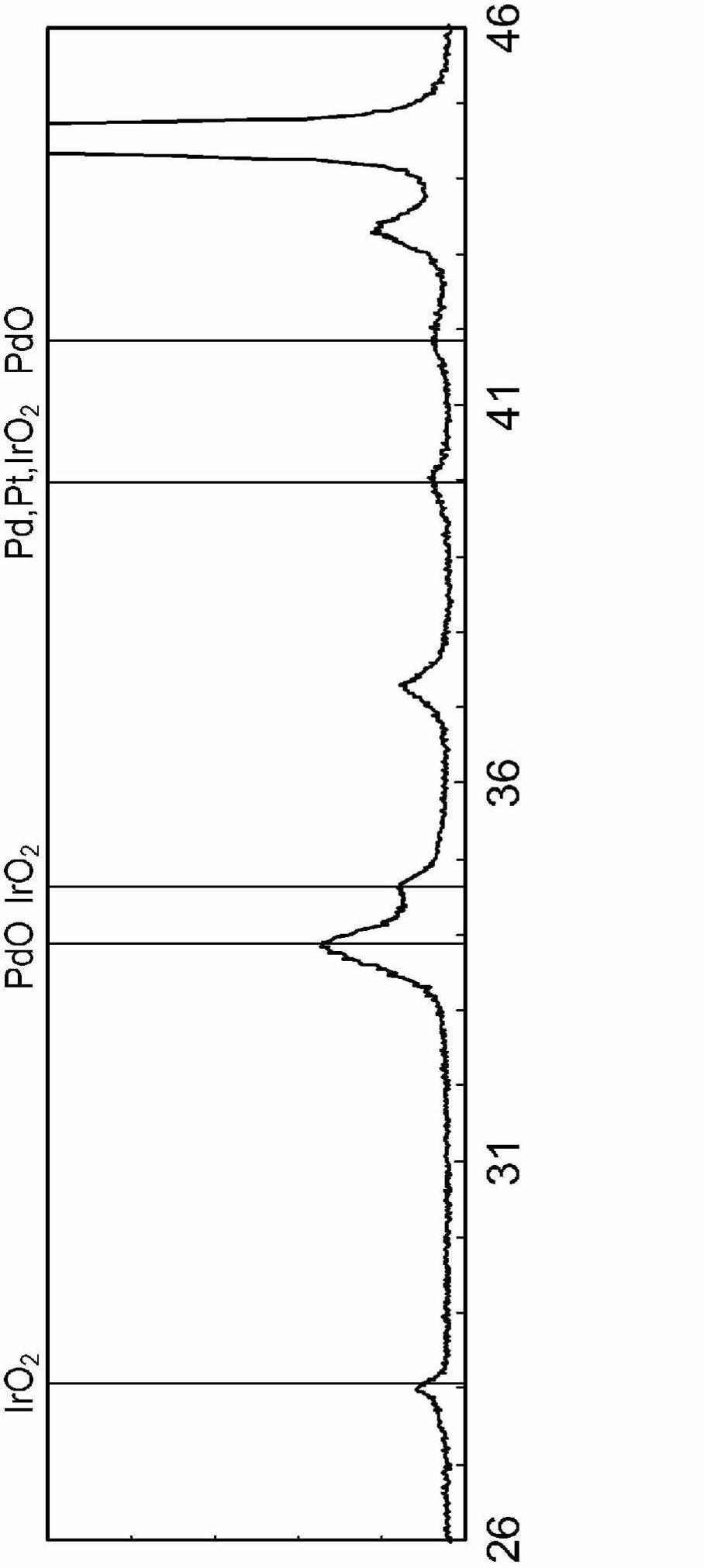
[0165] As a conductive base material, a mesh base material obtained by weaving nickel thin wires with a diameter of 0.15 mm in a 40-mesh mesh was used. Sandblasting was performed using alumina powder having a weight average particle diameter of 100 μm or less, followed by acid treatment in 6N hydrochloric acid at room temperature for 5 minutes, followed by water washing and drying.
[0166] Next, palladium nitrate solution (manufactured by Tanaka Kikinzoku, palladium concentration: 100 g / L) and dinitrosodiammine platinum nitric acid solution (manufactured by Tanaka Kikinzoku, platinum concentration: 100 g / L) were mixed so that the molar ratio of palladium and platinum was 1. 1. Prepare the first coating solution.
[0167] At the bottom of the coating roller (roll), a tray with the above-mentioned first coating solution is installed, the coating solution is infiltrated into the coating roller made of EPDM, and a roller is installed on the upper part so that the roller and the c...
Embodiment 2
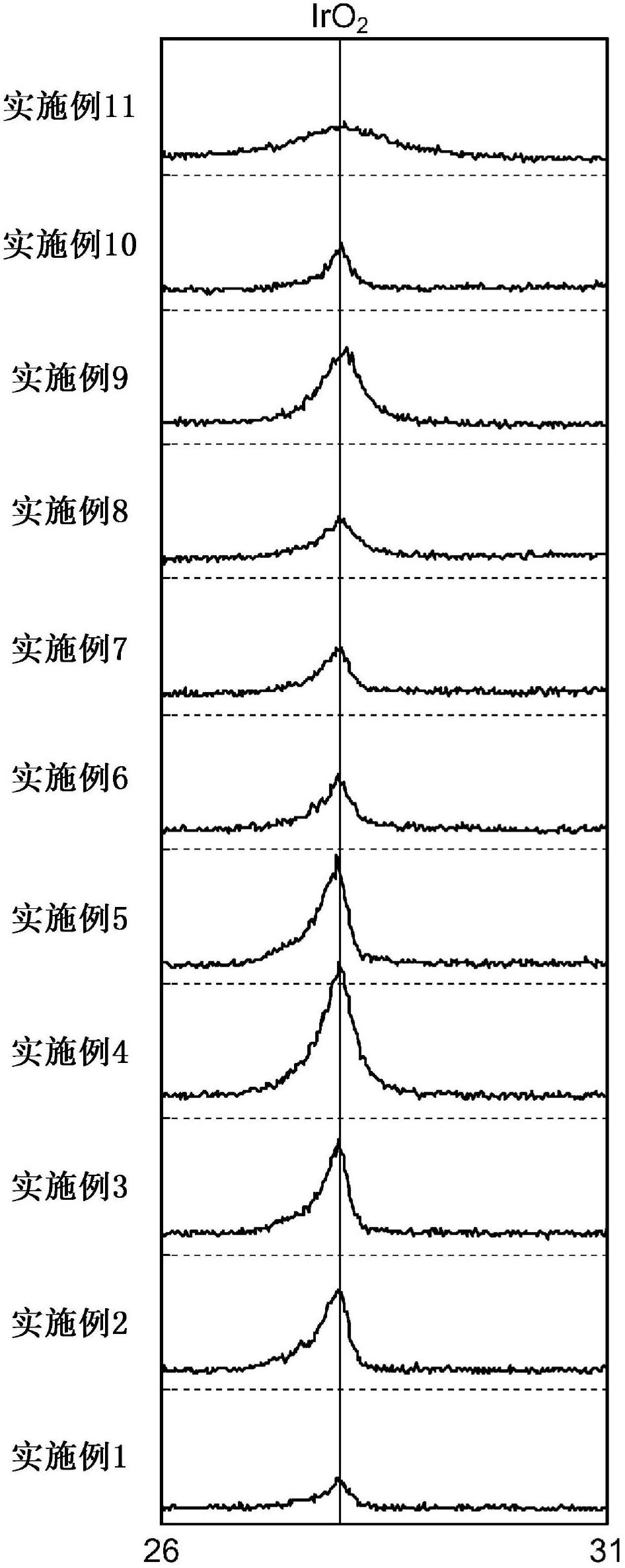
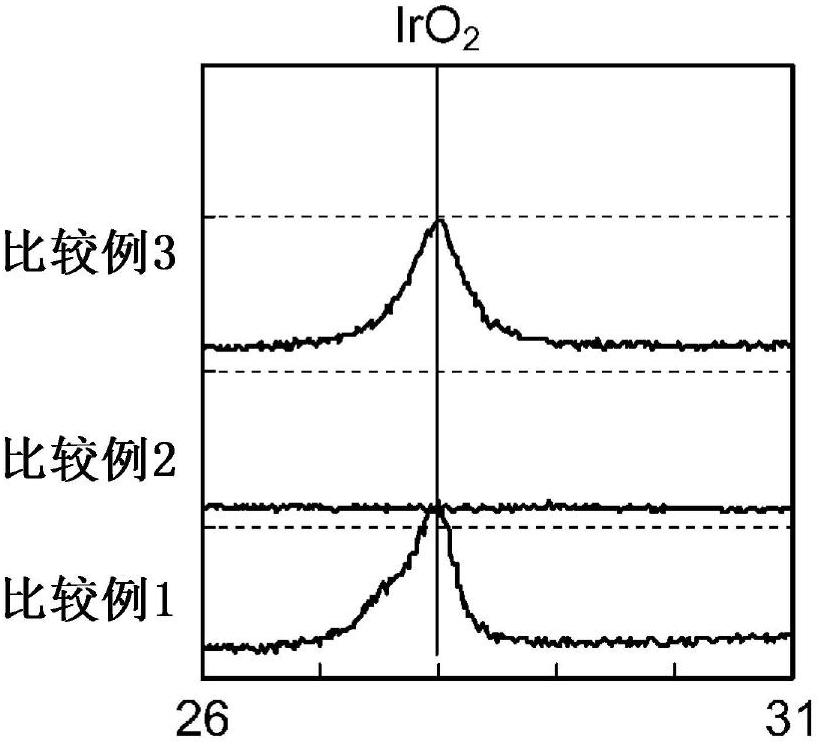
[0171] A cathode was prepared and evaluated in the same manner as in Example 1, except that the number of times of coating of the first layer was adjusted to one, and the number of times of coating of the second layer was adjusted to four. The amount of platinum contained in this test cathode was about 1.03 times that of the test cathode of Example 1.
[0172] As shown in Table 1, the results of the ion exchange membrane method salt electrolysis test are, 4kA / m 2 The hydrogen overvoltage under the condition is 92mV, and a cathode with low hydrogen overvoltage is obtained. As a result of the reverse current resistance test, the amount of platinum in the cathode after the test was 97% of the platinum amount before the test, and a cathode with strong reverse current resistance was obtained.
Embodiment 3
[0174] A cathode was fabricated and evaluated in the same manner as in Example 1, except that the number of times of coating of the first layer was adjusted to 2, and the number of times of coating of the second layer was adjusted to 4. The amount of platinum contained in this test cathode was about 1.39 times that of the test cathode of Example 1.
[0175] As shown in Table 1, the results of the ion exchange membrane method salt electrolysis test are, 4kA / m 2 The hydrogen overvoltage under the condition is 94mV, and a cathode with low hydrogen overvoltage is obtained. As a result of the reverse current resistance test, the amount of platinum in the cathode after the test was 96% of the platinum amount before the test, and a cathode with strong reverse current resistance was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com