Novel pyrimidine-fused cyclic compounds as cytokine inhibitors
A compound, solvate technology, applied in a series of diseases, alleviating and controlling various diseases, treatment, prevention field, able to solve problems such as immune system errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
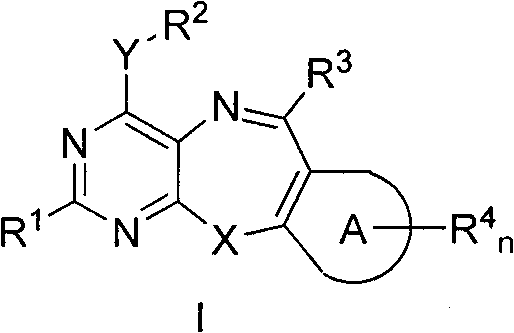
[0289] Example 1: Pyrimido[4,5-b][1,4]benzothiazepine Synthesis of derivatives Synthesis experimental steps:
[0290]
[0291] The synthesis of precursor material 1 refers to published papers and patents (Bai, X. et, al. J. Org. Chem., 2005, 70, 10810. and PCT / US2006 / 006303).
[0292] pyrimido[4,5-b][1,4]benzothiazepine Synthesis of sulfoxide:
[0293]
[0294] 4-phenylthio-6-phenylpyrimido[4,5-b][1,4]benzothiazepine 1.0 (1.0mmol) dissolved in CH 2 Cl 2 (10mL), under magnetic stirring at room temperature, slowly drop m-CPBA (2.2mmol) in CH 2 Cl 2 (15mL) solution, used for 1 hour, continued to react for 3 hours after dripping. The reaction solution was sequentially washed with saturated NaHSO 3 , saturated Na 2 CO 3 Wash with saturated NaCl solution, Na 2 SO 4 Drying, evaporation of the solvent under reduced pressure, separation on a silica gel column (petroleum ether / ethyl acetate=5:1, v / v) gave 4-phenylsulfinyl-6-phenylpyrimido[4,5-b][1 , 4] benzothiazep...
Embodiment 2
[0357] Example 2: p38 Kinase Inhibition - In Vitro Assay
[0358] Experimental method: Kinase assay adopts the SelectScreen of Invitrogen Corporation (501 Charmany Drive, Madison, Wisconsin, 53719) TM Kinase Profiling Service conducted. The specific experimental steps are as follows: the compound is dissolved in DMSO to prepare a 100x mother solution. Then with kinase buffer (50mM HEPES, pH 7.5, 0.01% BRIJ-35, 10mM MgCl 2 , 1mM EGTA) to make a 4x working solution, take 2.5μL and add it to a 384-well plate, set up a 0 inhibition control and a 100% inhibition control, and then add 5μL to the well to prepare 2x MAPK14(p38α), inactivation MAPKAPK2 and Ser / Thr04 peptide mixture, and then add 2.5μL of 4x ATP solution diluted with kinase buffer to each well to form a 10μL reaction system, shake the plate for 30s, and incubate at room temperature for 1h; then add 5μL of developing solution (1:1024 dilution), shake the plate for 30s, incubate at room temperature for 1h, detect and ...
Embodiment 3
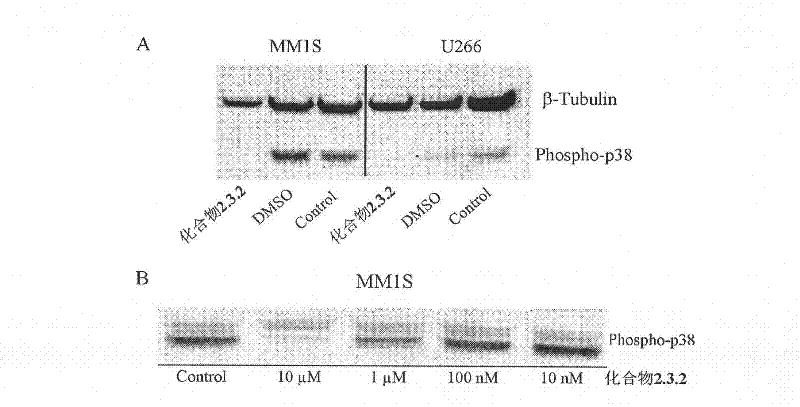
[0361] Example 3: Cell model inhibits p38α phosphorylation test
[0362] Cells used in the experiment: MM1S cell line, U266 cell line
[0363] Test method: MM1S cell line and U266 cell line are both at 37°C, 5% CO 2 1640 medium was used for cultivation under the conditions, and 1640 medium contained 10% calf serum, 1% double antibody, and 1% L-glutamine. Dissolve compound 2.3.2 in DMSO to make a solution with a concentration of 10 mM, and use 1640 medium to prepare a solution with a concentration of 10 μM, which acts on MM1S cells and U266 cells respectively. Separately set up a solvent group and a control group, at 37 ° C, 5% CO 2 Cultivate in the incubator for 3 hours, collect the cells, extract the protein, and detect the effect of the compound on p38α phosphorylation by Western blot kit (Cell Signaling Antibody, cat.#9211); at the same time, compound 2.3.2 was formulated into 10 μM, 1 μM, 0.1 μM , 0.01 μM and other different concentrations (the content of DMSO is 0.2%),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com