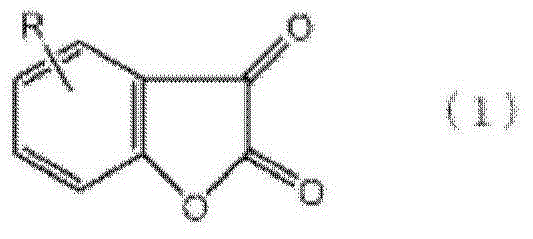
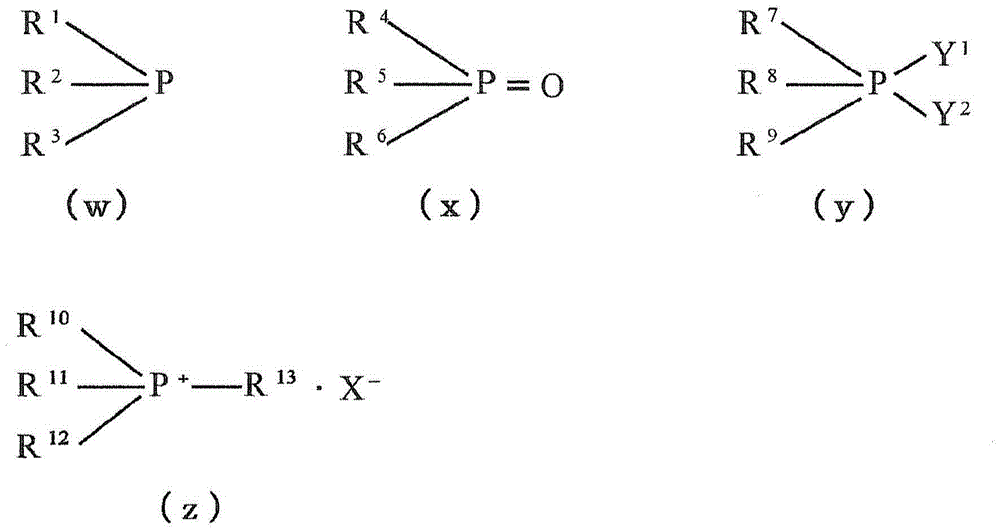The manufacture method of diaryl oxalate
A technology for diphenyl oxalate and dialkyl oxalate, which is applied in the field of manufacturing diaryl oxalate, can solve the problems of increased impurities, reduced selectivity, difficulty in manufacturing and the like, and achieves improved selectivity, improved reaction efficiency, The effect of manufacturing equipment simplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0192] The present invention is illustrated in detail by way of examples below. However, the present invention is not limited to the following examples at all.
Synthetic example 1
[0194] Synthesis of tetraphenoxytitanium
[0195] 300 ml of phenol was charged at the bottom of a flat-bottomed flask with an inner diameter of 32 mm and an Oldershaw type reactive distillation column (hereinafter referred to as "reactive distillation column for catalyst synthesis") having a volume of 1 L, and heated with an oil bath to raise the temperature. After the temperature distribution in the reactive distillation tower for catalyst synthesis was stabilized while maintaining the total reflux state, phenol was fed at a flow rate of 342.4g / h, and titanium tetraisopropoxide (TIPT) was fed at a flow rate of 21.6g / h . The ratio of the number of moles of phenol fed to the number of moles of Ti atoms in the TIPT (phenol / Ti) was 48. Keeping the reflux ratio at 5 the distillate from the top was withdrawn. In addition, it was continuously withdrawn from the bottom of the reaction distillation column for catalyst synthesis so that the liquid level at the bottom was maintained a...
Synthetic example 2~4
[0200] Synthesis of tetraphenoxytitanium
[0201] The same experiment as in Synthesis Example 1 was performed by changing some of the conditions shown in Table 1, and the results are shown in Table 1.
[0202] In addition, in the reaction solution of Synthesis Example 4, TPT was partially separated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com