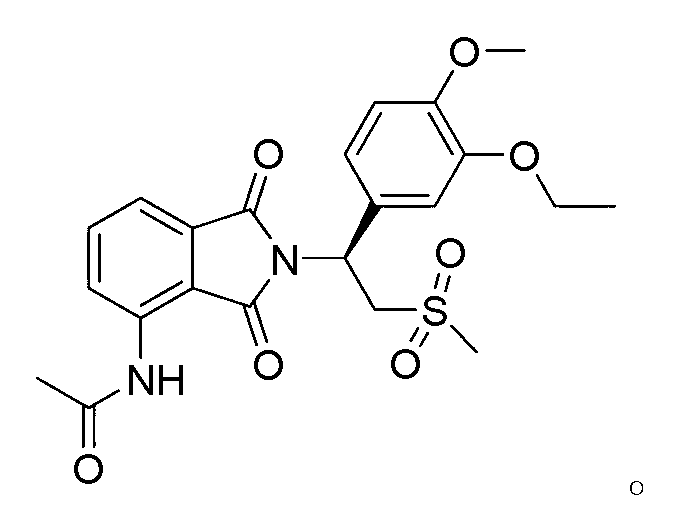
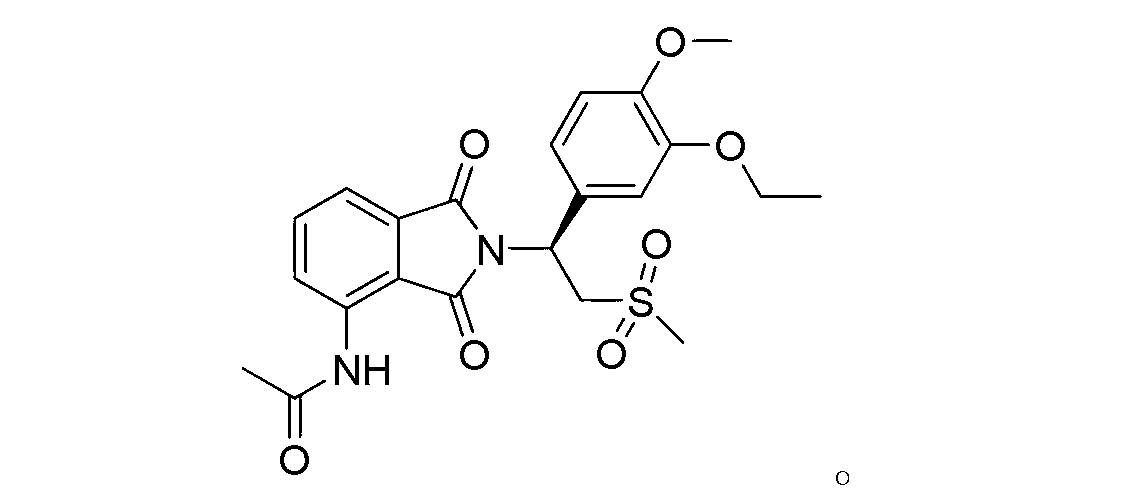
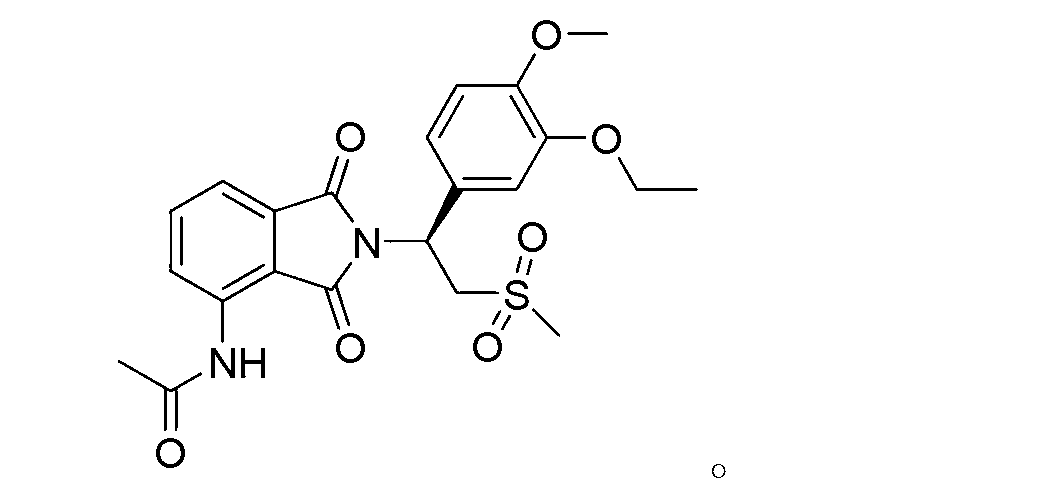
Apremilast For The Treatment Of Sarcoidosis
A technology for sarcoidosis, nodules, applied in the field of pharmaceutical compositions and dosing regimens, capable of solving difficult standard therapy, treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The present invention provides methods of treating, controlling or preventing sarcoidosis comprising administering a PDE4 inhibitor to a patient in need of such treatment, managing or preventing. In one embodiment, the sarcoidosis is acute sarcoidosis. In other embodiments, the sarcoidosis is chronic sarcoidosis.
[0018] In one embodiment, the sarcoidosis includes, but is not limited to: cardiac sarcoidosis, skin sarcoidosis, hepatic sarcoidosis, oral sarcoidosis, pulmonary sarcoidosis, nervous system sarcoidosis, sinonasal nodules sarcoidosis, Lofgren's syndrome, lupus peritoneum, uveitis, or chronic cutaneous sarcoidosis.
[0019] In one embodiment, the sarcoidosis is selected from the group consisting of: cardiac sarcoidosis, cutaneous sarcoidosis, hepatic sarcoidosis, oral sarcoidosis, pulmonary sarcoidosis, nervous system sarcoidosis, nasal sinus sarcoidosis , Lofgren's syndrome, lupus pereptoid, uveitis, or chronic cutaneous sarcoidosis.
[0020] In one embodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com